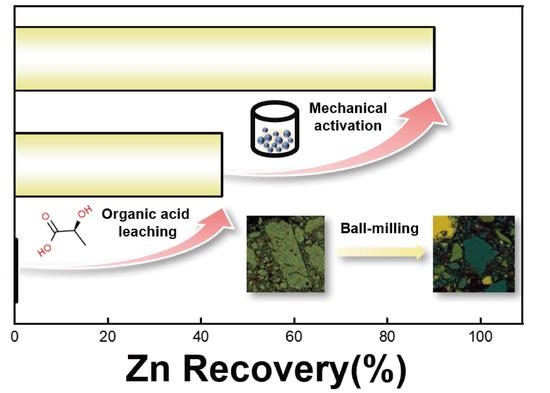
Enhanced Recovery of Zn from Carbonate-Type Mixed Oxidized Ore (CMO) by Combining Organic Acid Leaching with Mechanical Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineralogical Characterization
2.2. Experimental Procedures
2.2.1. Leaching Methods
2.2.2. Analysis Method
2.2.3. Experimental Design for Leaching Condition Optimization
2.2.4. Mechanical Activation Treatment
3. Results and Discussion
3.1. Characteristics of CMO
3.2. Zn Leaching Effects of Organic Acids
3.3. Leaching Condition Optimization
3.3.1. pH Optimization
3.3.2. RSM Model Fitting, Evaluation, and Confirmation
3.3.3. Interactions among Factors and Optimization
3.3.4. Optimization and Comparison of Zn Leaching between the Predicted and Actual Value at Optimized Condition
3.4. Combined Effect of Mechanical Activation on Zn Leaching
3.4.1. Effect of Rotation Speed
3.4.2. Effect of Ball-Mass Ratio
3.4.3. Effect of Rotation Time
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaya, M.; Hussaini, S.; Kursunoglu, S. Critical review on secondary zinc resources and their recycling technologies. Hydrometallurgy 2020, 195, 105362. [Google Scholar] [CrossRef]
- Reuter, M.A.; Worrell, E. Handbook of Recycling; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Xia, Z.; Zhang, X.; Huang, X.; Yang, S.; Chen, Y.; Ye, L. Hydrometallurgical stepwise recovery of copper and zinc from smelting slag of waste brass in ammonium chloride solution. Hydrometallurgy 2020, 197, 105475. [Google Scholar] [CrossRef]
- Lupi, C.; Pilone, D.; Cavallini, M. Hydrometallurgical processing of EAF steelmaking fumes. In Proceedings of the Second International Symposium on Extraction and Processing for the Treatment and Minimization of Wastes 1996, Phoenix, AZ, USA, 27–30 October 1996; pp. 711–718. [Google Scholar]
- Liu, Q.; Zhao, Y.; Zhao, G. Production of zinc and lead concentrates from lean oxidized zinc ores by alkaline leaching followed by two-step precipitation using sulfides. Hydrometallurgy 2011, 110, 79–84. [Google Scholar] [CrossRef]
- Liu, W.; Huang, F.; Liao, Y.; Zhang, J.; Ren, G.; Zhuang, Z.; Zhen, J.; Lin, Z.; Wang, C. Treatment of CrVI -Containing Mg(OH)2 Nanowaste. Angew. Chem. Int. Ed. 2008, 47, 5619–5622. [Google Scholar] [CrossRef] [PubMed]
- Dutra, A.J.B.; Paiva, P.R.P.; Tavares, L.M. Alkaline leaching of zinc from electric arc furnace steel dust. Miner. Eng. 2005, 19, 478–485. [Google Scholar] [CrossRef]
- Pathak, A.; Vinoba, M.; Kothari, R. Emerging role of organic acids in leaching of valuable metals from refinery-spent hydroprocessing catalysts, and potential techno-economic challenges: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1–43. [Google Scholar] [CrossRef]
- Halli, P.; Hamuyuni, J.; Leikola, M.; Lundström, M. Developing a sustainable solution for recycling electric arc furnace dust via organic acid leaching. Miner. Eng. 2018, 124, 1–9. [Google Scholar] [CrossRef]
- Gharabaghi, M.; Irannajad, M.; Noaparast, M. A review of the beneficiation of calcareous phosphate ores using organic acid leaching. Hydrometallurgy 2010, 103, 96–107. [Google Scholar] [CrossRef]
- Tiechui, Y.; Qinyuan, C.; Jie, L. Effects of mechanical activation on physicochemical properties and alkaline leaching of hemimorphite. Hydrometallurgy 2010, 104, 136–141. [Google Scholar] [CrossRef]
- Guo, L.; Hu, Z.; Du, Y.; Zhang, T.C.; Du, D. Mechanochemical activation on selective leaching of arsenic from copper smelting flue dusts. J. Hazard. Mater. 2021, 414, 125436. [Google Scholar] [CrossRef]
- Ashtari, P.; Pourghahramani, P. Selective mechanochemical alkaline leaching of zinc from zinc plant residue. Hydrometallurgy 2015, 156, 165–172. [Google Scholar] [CrossRef]
- Mirazimi, S.M.J.; Rashchi, F.; Saba, M. Vanadium removal from roasted LD converter slag: Optimization of parameters by response surface methodology (RSM). Sep. Purif. Technol. 2013, 116, 175–183. [Google Scholar] [CrossRef]
- Choi, J.-W.; Cho, C.-W.; Yun, Y.-S. Organic acid-based linear free energy relationship models for green leaching of strategic metals from spent lithium-ion batteries and improvement of leaching performance. J. Hazard. Mater. 2022, 423, 127214. [Google Scholar] [CrossRef]
- Dong, J.; Liu, Q.; Subhonqulov, S.H.; Sheng, J.; Gao, Y.; Liu, M. Research on the flotation of sphalerite and germanium-bearing sphalerite activated by copper ion and its mechanism difference. Miner. Eng. 2022, 186, 107756. [Google Scholar] [CrossRef]
- Li, L.; Ge, J.; Wu, F.; Chen, R.; Chen, S.; Wu, B. Recovery of cobalt and lithium from spent lithium ion batteries using organic citric acid as leachant. J. Hazard. Mater. 2010, 176, 288–293. [Google Scholar] [CrossRef]
- Burckhard, S.R.; Schwab, A.P.; Banks, M.K. The effects of organic acids on the leaching of heavy metals from mine tailings. J. Hazard. Mater. 1995, 41, 135–145. [Google Scholar] [CrossRef]
- Yunda, E.; Godymchuk, A. Dissolution of zinc nanoparticles in pulmonary fluid. In Proceedings of the 2012 7th International Forum on Strategic Technology (IFOST), Tomsk, Russia, 18–21 September 2012; pp. 1–4. [Google Scholar]
- Choi, J.-W.; Kim, J.; Kim, S.-K.; Yun, Y.-S. Simple, green organic acid-based hydrometallurgy for waste-to-energy storage devices: Recovery of NiMnCoC2O4 as an electrode material for pseudocapacitor from spent LiNiMnCoO2 batteries. J. Hazard. Mater. 2022, 424, 127481. [Google Scholar] [CrossRef]
- Abraham, M.H.; Zhao, Y.H. Determination of Solvation Descriptors for Ionic Species: Hydrogen Bond Acidity and Basicity. J. Org. Chem. 2004, 69, 4677–4685. [Google Scholar] [CrossRef]
- Banerjee, R.; Mohanty, A.; Chakravarty, S.; Chakladar, S.; Biswas, P. A single-step process to leach out rare earth elements from coal ash using organic carboxylic acids. Hydrometallurgy 2021, 201, 105575. [Google Scholar] [CrossRef]
- Ke, Z.-B.; Fan, X.-H.; You-Ying, D.; Chen, F.-Y.; Zhang, L.-J.; Yang, K.; Li, B.; Kong, Y.-X. Crystal structure and solution thermodynamics of the lactate complex Zn[(C3H5O3)2(H2O)2]⋅H2O(s). Chem. Thermodyn. Therm. Anal. 2022, 5, 100024. [Google Scholar] [CrossRef]
- James Speight, P.D. Lange’s Handbook of Chemistry, Sixteenth Edition, 16th ed.; McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Fest, E.P.M.J.; Temminghoff, E.J.M.; Griffioen, J.; Van Riemsdijk, W.H. Proton Buffering and Metal Leaching in Sandy Soils. Environ. Sci. Technol. 2005, 39, 7901–7908. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Ding, X.; Cai, C.; Peng, G.; Hu, J.; Yang, X.; Chen, S.; Liu, L.; Hou, H.; Liang, S.; et al. Acetate acid and glucose assisted subcritical reaction for metal recovery from spent lithium ion batteries. J. Clean. Prod. 2022, 369, 133281. [Google Scholar] [CrossRef]
- Li, P.; Luo, S.-H.; Su, F.; Zhang, L.; Yan, S.; Lei, X.; Mu, W.; Wang, Q.; Zhang, Y.; Liu, X.; et al. Optimization of Synergistic Leaching of Valuable Metals from Spent Lithium-Ion Batteries by the Sulfuric Acid-Malonic Acid System Using Response Surface Methodology. ACS Appl. Mater. Interfaces 2022, 14, 11359–11374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiang, J.; Pei, G.; Li, L.; Huang, Q.; Lv, X. Application of response surface methodology for roasting optimization in composite roasting—Acid leaching vanadium extraction process. Chem. Eng. Res. Des. 2021, 172, 254–263. [Google Scholar] [CrossRef]
- Zhang, Y.; Muhammed, M. The removal of phosphorus from iron ore by leaching with nitric acid. Hydrometallurgy 1989, 21, 255–275. [Google Scholar] [CrossRef]
- Clarholm, M.; Skyllberg, U.; Rosling, A. Organic acid induced release of nutrients from metal-stabilized soil organic matter–The unbutton model. Soil Biol. Biochem. 2015, 84, 168–176. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, Y.; Liu, T.; Hu, P.; Zheng, Q. Optimization of microwave roasting-acid leaching process for vanadium extraction from shale via response surface methodology. J. Clean. Prod. 2019, 234, 494–502. [Google Scholar] [CrossRef]
- Pourghahramani, P.; Forssberg, E. Microstructure characterization of mechanically activated hematite using XRD line broadening. Int. J. Miner. Process. 2006, 79, 106–119. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Winiarska, K.; Szczygieł, I.; Szczygiel, B. XPS and FT-IR Characterization of Selected Synthetic Corrosion Products of Zinc Expected in Neutral Environment Containing Chloride Ions. J. Spectrosc. 2018, 2018, 2079278. [Google Scholar] [CrossRef]
- Catalano, M.; Belluso, E.; Miriello, D.; Barrese, E.; Bloise, A. Synthesis of Zn-doped talc in hydrothermal atmosphere. Cryst. Res. Technol. 2014, 49, 283–289. [Google Scholar] [CrossRef]
- Dudev, T.; Lim, C. Tetrahedral vs Octahedral Zinc Complexes with Ligands of Biological Interest: A DFT/CDM Study. J. Am. Chem. Soc. 2000, 122, 11146–11153. [Google Scholar] [CrossRef]
- Kodali, P.; Dhawan, N.; Depci, T.; Lin, C.L.; Miller, J.D. Particle damage and exposure analysis in HPGR crushing of selected copper ores for column leaching. Miner. Eng. 2011, 24, 1478–1487. [Google Scholar] [CrossRef]
- Hussaini, S.; Tita, A.M.; Kursunoglu, S.; Top, S.; Ichlas, Z.T.; Kar, U.; Kaya, M. Pb-Zn recovery from a malic leach solution of a carbonate type ore flotation tailing by precipitation and solvent extraction. Sep. Purif. Technol. 2021, 272, 118963. [Google Scholar] [CrossRef]
- Kursunoglu, S.; Kursunoglu, N.; Hussaini, S.; Kaya, M. Selection of an appropriate acid type for the recovery of zinc from a flotation tailing by the analytic hierarchy process. J. Clean. Prod. 2021, 283, 124659. [Google Scholar] [CrossRef]
- Jia, N.; Wang, H.-g.; Zhang, M.; Guo, M. Selective and Efficient Extraction of Zinc from Mixed Sulfide–oxide Zinc and Lead Ore. Miner. Process. Extr. Metall. Rev. 2016, 37, 418–426. [Google Scholar] [CrossRef]
- Khodaei, H.; Fatmehsari Haghshenas, D.; Firoozi, S. Selective leaching of zinc from carbonate source using glycine as an ecofriendly lixiviant. Miner. Eng. 2022, 185, 107680. [Google Scholar] [CrossRef]
- Hussaini, S.; Kursunoglu, S.; Top, S.; Ichlas, Z.T.; Kaya, M. Testing of 17-different leaching agents for the recovery of zinc from a carbonate-type Pb-Zn ore flotation tailing. Miner. Eng. 2021, 168, 106935. [Google Scholar] [CrossRef]
- Halli, P.; Hamuyuni, J.; Revitzer, H.; Lundström, M. Selection of leaching media for metal dissolution from electric arc furnace dust. J. Clean. Prod. 2017, 164, 265–276. [Google Scholar] [CrossRef]
- Meng, F.; Liu, Q.; Kim, R.; Wang, J.; Liu, G.; Ghahreman, A. Selective recovery of valuable metals from industrial waste lithium-ion batteries using citric acid under reductive conditions: Leaching optimization and kinetic analysis. Hydrometall. 2020, 191, 105160. [Google Scholar] [CrossRef]
- Diebler, H.E. (Ed.) Stability Constants of Metal-Ion Complexes, Part A: Inorganic Ligands, Vol. 21 aus: IUPAC Chemical Data Series. Pergamon Press, Oxford, New York, Toronto, Sydney, Paris, Frankfurt 1982. 310 Seiten, Preis: $ 85.00. Berichte der Bunsengesellschaft für physikalische Chemie 1983, 87, 1227. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, X.; Teeter, G.; To, B.; Yan, Y.; Dhere, R.G.; Gessert, T.A. CBD-Cd1−xZnxS thin films and their application in CdTe solar cells. Phys. Status Solidi (B) 2004, 241, 775–778. [Google Scholar] [CrossRef]





| Element | Zn | Pb | Fe | S | Al | Ca | Mg | Others |
|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 28.6 | 14.9 | 10.5 | 16.9 | 0.542 | 3.10 | 0.512 | 25.5 |
| pKa 1 | lgK1(Zn) 2 | Solubility of Zn-Complex 2 | lgK1(Fe) 2 | lgK1(Pb) 2 | |
|---|---|---|---|---|---|
| lactic acid | 3.78 | 2.20 | Soluble | 7.10(3+) | 2.40 |
| malonic acid | 2.43 | - | Soluble | - | - |
| citric acid | 3.06 | 4.71(HL2-) | Slightly soluble | 3.08(2+) 12.5(3+) | 6.50 |
| amber acid (succinic acid) | 3.55 | 1.60 | Soluble | 7.49(3+) | 2.80 |
| acetic acid | 4.54 | 1.50 | 30.0 g/100g saturated solution | 3.20(2+) | 2.52 |
| tartaric acid | 2.72 | 2.68 | 0.0220 g/100 mL | 7.49(3+) | 3.78 |
| pH | Lactic Acid Concentration | Temperature | Time | L/S | Zn Leaching Efficiency (%) | |
|---|---|---|---|---|---|---|
| 1 | (mol/L) | (°C) | (min) | Predicted | Experimental | |
| 2 | 1.15 | 75 | 75 | 20 | 46.5 | 44.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, H.; Pan, X.; Chen, F.; Gao, Q.; Tian, C.; Lin, Z. Enhanced Recovery of Zn from Carbonate-Type Mixed Oxidized Ore (CMO) by Combining Organic Acid Leaching with Mechanical Activation. Metals 2023, 13, 1021. https://doi.org/10.3390/met13061021
Deng H, Pan X, Chen F, Gao Q, Tian C, Lin Z. Enhanced Recovery of Zn from Carbonate-Type Mixed Oxidized Ore (CMO) by Combining Organic Acid Leaching with Mechanical Activation. Metals. 2023; 13(6):1021. https://doi.org/10.3390/met13061021
Chicago/Turabian StyleDeng, Hao, Xuelin Pan, Fanyun Chen, Qingshan Gao, Chen Tian, and Zhang Lin. 2023. "Enhanced Recovery of Zn from Carbonate-Type Mixed Oxidized Ore (CMO) by Combining Organic Acid Leaching with Mechanical Activation" Metals 13, no. 6: 1021. https://doi.org/10.3390/met13061021