Superior Plasticity of Silver-Based Composites with Reinforcing Pyrochlore
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Synthesis
2.2.1. Synthesis of La2Sn2O7 and SnO2 Powders
2.2.2. Composite Fabrication
2.3. Material Characterization
2.4. Tensile Test
2.5. Computational Method
3. Results
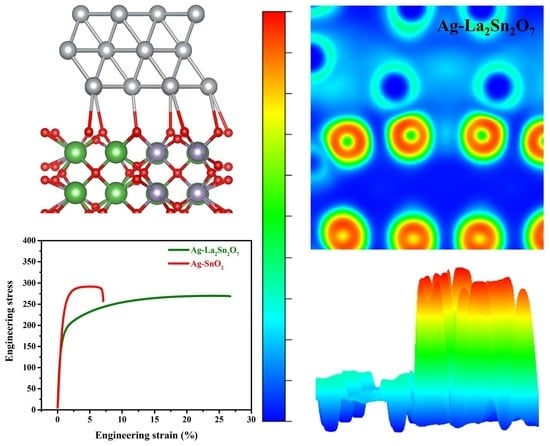
3.1. Theoretical Evaluation of Interfacial Bonding Strength
3.2. Microstructures of the Ag-SnO2 and Ag-La2Sn2O7 Composites
3.3. Tensile Test
4. Discussion
4.1. Experimental Demonstration of Enhanced Interface Bonding Strength
4.2. Mechanism of Enhanced Interface Bonding Strength
4.3. Geometric Phase Analysis (GPA) around the Interfaces
4.4. Enhanced Interfacial Mobility and Plasticity
5. Conclusions
- (1)
- The as-prepared Ag-La2Sn2O7 composite showed enhanced plasticity, approximately 2.8 times that of the traditional and widely used Ag-SnO2 composite.
- (2)
- The good conformity between the experimental conductivity and the theoretical conductivity of the Ag-La2Sn2O7 composites indicated interfaces with less defects, which can scatter moving electrons, and good interfacial bonding strength, which hinders the formation of cracks.
- (3)
- The enhanced mobility of the Ag-La2Sn2O7 interface helped to release the stress concentration at the interface, inhibiting the formation and growth of microcracks and the debonding phenomenon.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahiraei, M.; Heshmatian, S. Thermal performance and second law characteristics of two new microchannel heat sinks operated with hybrid nanofluid containing graphene–silver nanoparticles. Energ. Convers. Manag. 2018, 168, 357–370. [Google Scholar] [CrossRef]
- Damle, T.; Varenberg, M.; Graber, L. Electric Contact Material Selection for Medium and High Voltage DC Circuit Breakers. Trans. Electr. Electron. Mater. 2020, 21, 329–338. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Cai, F.; Zhong, S.; Ma, J.; Bao, L.; Jiu, Y.; Hu, B.; Wei, S.; Long, W. Microstructure and mechanical properties of brazing joint of silver-based composite filler metal. Nanotechnol. Rev. 2020, 9, 1034–1043. [Google Scholar] [CrossRef]
- Kennes, K.; Martin, C.; Baekelant, W.; Coutino-Gonzalez, E.; Fron, E.; Roeffaers, M.; Hofkens, J.; Van der Auweraer, M. Silver Zeolite Composite-Based LEDs: Origin of Electroluminescence and Charge Transport. ACS Appl. Mater. Inter. 2019, 11, 12179–12183. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, D.; Wang, Y. Microstructure and properties of Ag–SnO2 materials with high SnO2 content. J. Alloy. Compd. 2014, 582, 1–5. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, L.; Yang, H.; Shen, T.; Chen, L. Effect of preparing method of ZnO powders on electrical arc erosion behavior of Ag/ZnO electrical contact material. J. Mater. Res. 2016, 31, 468–479. [Google Scholar] [CrossRef]
- Hetzmannseder, E.; Rieder, W. Make-and-break erosion of Ag/MeO contact materials. IEEE Trans. Compon. Packag. Manuf. Technol. Part A 1996, 19, 397–403. [Google Scholar] [CrossRef]
- Muñoz-Morris, M.A.; Garcia Oca, C.; Morris, D.G. An analysis of strengthening mechanisms in a mechanically alloyed, oxide dispersion strengthened iron aluminide intermetallic. Acta Mater. 2002, 50, 2825–2836. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, L.; Shen, T.; Qiao, Z.; Yang, H.; Fan, X.; Chen, L. Effects of Oxide-Modified Spherical ZnO on Electrical Properties of Ag/ZnO Electrical Contact Material. J. Mater. Eng. Perform. 2016, 25, 3662–3671. [Google Scholar] [CrossRef]
- Liu, G.; Wang, S.; Misra, A.; Wang, J. Interface-mediated plasticity of nanoscale Al–Al2Cu eutectics. Acta Mater. 2020, 186, 443–453. [Google Scholar] [CrossRef]
- Lou, M.; Chen, X.; Xu, K.; Deng, Z.; Chen, L.; Lv, J.; Chang, K.; Wang, L. Temperature-induced wear transition in ceramic-metal composites. Acta Mater. 2021, 205, 116545. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Norskov, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T. Density functional theory in surface chemistry and catalysis. PNAS 2011, 108, 937–943. [Google Scholar] [CrossRef]
- Xilian, S.; Jianda, S. Influence of Cr interlayer on the structure and optical properties of Ag films on glass substrate by magnetron sputtering. Appl. Surf. Sci. 2006, 253, 2093–2095. [Google Scholar] [CrossRef]
- Russell, S.; Rafalski, S.; Spreitzer, R.; Li, J.; Moinpour, M.; Moghadam, F.; Alford, T. Enhanced adhesion of copper to dielectrics via titanium and chromium additions and sacrificial reactions. Thin Solid Films 1995, 262, 154–167. [Google Scholar] [CrossRef]
- Hou, C.; Song, X.; Tang, F.; Li, Y.; Cao, L.; Wang, J.; Nie, Z. W–Cu composites with submicron- and nanostructures: Progress and challenges. NPG Asia Mater. 2019, 11, 74. [Google Scholar] [CrossRef]
- Matsunaka, D.; Shibutani, Y. Effects of oxygen vacancy on adhesion of incoherent metal/oxide interface by first-principles calculations. Surf. Sci. 2010, 604, 196–200. [Google Scholar] [CrossRef]
- Ferrari, A.; Pacchioni, G. Metal Deposition on Oxide Surfaces: A Quantum-Chemical Study of the Interaction of Rb, Pd, and Ag Atoms with the Surface Vacancies of MgO. J. Phys. Chem. 1996, 100, 9032–9037. [Google Scholar] [CrossRef]
- Liu, Y.; Ning, X. Influence of α-Al2O3 (0001) surface reconstruction on wettability of Al/Al2O3 interface: A first-principle study. Comp. Mater. Sci. 2014, 85, 193–199. [Google Scholar] [CrossRef]
- Jikihara, A.; Tanaka, C.; Ballester, R.; Meira, J. Zirconia-ceramic versus metal-ceramic: Thermal expansion mismatch and residual stresses. Dent. Mater. 2018, 34, e62–e63. [Google Scholar] [CrossRef]
- Levy-Tubiana, R.; Baczmanski, A.; Lodini, A. Relaxation of thermal mismatch stress due to plastic deformation in an Al/SiCp metal matrix composite. Mater. Sci. Eng. A 2003, 341, 74–86. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W. Thermal expansion and conductivity of RE2Sn2O7 (RE = La, Nd, Sm, Gd, Er and Yb) pyrochlores. Scr. Mater. 2013, 69, 401–404. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics liquid-metal—Amorphous-semiconductor simulation of the transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Blöchl, P.; Jepsen, O.; Andersen, O. Improved tetrahedron method far Brilleuin-zane integratians. Phys. Rev. B 1994, 49, 16223–16233. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Roundy, D.; Krenn, C.R.; Cohen, M.L.; Morris, J.W. Ideal Shear Strengths of fcc Aluminum and Copper. Phys. Rev. Lett. 1999, 82, 2713–2716. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2014, 14, 23–36. [Google Scholar] [CrossRef]
- Yang, P.; Li, Q.; Tsuru, T.; Ogata, S.; Zhang, J.; Sheng, H.; Shan, Z.; Sha, G.; Han, W.; Li, J.; et al. Mechanism of hardening and damage initiation in oxygen embrittlement of body-centred-cubic niobium. Acta Mater. 2019, 168, 331–342. [Google Scholar] [CrossRef]
- Rong, X.; Zhao, D.; He, C.; Shi, C.; Liu, E.; Zhao, N. Revealing the strengthening and toughening mechanisms of Al-CuO composite fabricated via in-situ solid-state reaction. Acta Mater. 2021, 204, 116524. [Google Scholar] [CrossRef]
- Zhang, J.; Han, W. Oxygen solutes induced anomalous hardening, toughening and embrittlement in body-centered cubic vanadium. Acta Mater. 2020, 196, 122–132. [Google Scholar] [CrossRef]
- Choy, T. Effective Medium Theory; Oxford University Press: Britain, UK, 1999. [Google Scholar]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys.-Berl. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Verdozzi, C.; Jennison, D.R.; Schultz, P.A.; Sears, M.P. Sapphire (0001) Surface, Clean and with d-Metal Overlayers. Phys. Rev. Lett. 1999, 82, 799. [Google Scholar] [CrossRef]
- Bogicevic, A.; Jennison, D.R. Variations in the Nature of Metal Adsorption on Ultrathin Al2O3 Films. Phys. Rev. Lett. 1999, 82, 4050. [Google Scholar] [CrossRef]
- Petrie, J.R.; Cooper, V.R.; Freeland, J.W.; Meyer, T.L.; Zhang, Z.; Lutterman, D.A.; Lee, H.N. Enhanced Bifunctional Oxygen Catalysis in Strained LaNiO3 Perovskites. J. Am. Chem. Soc. 2016, 138, 2488–2491. [Google Scholar] [CrossRef]
- Duan, Y.; Sun, S.; Xi, S.; Ren, X.; Zhou, Y.; Zhang, G.; Yang, H.; Du, Y.; Xu, Z.J. Tailoring the Co 3d-O 2p Covalency in LaCoO3 by Fe Substitution to Promote Oxygen Evolution Reaction. Chem. Mater. 2017, 29, 10534–10541. [Google Scholar] [CrossRef]
- Kuznetsov, D.A.; Naeem, M.A.; Kumar, P.V.; Abdala, P.M.; Fedorov, A.; Müller, C.R. Tailoring Lattice Oxygen Binding in Ruthenium Pyrochlores to Enhance Oxygen Evolution Activity. J. Am. Chem. Soc. 2020, 142, 7883–7888. [Google Scholar] [CrossRef] [PubMed]
- Ribes, H.; Suéry, M.; L’Espérance, G.; Legoux, J.G. Microscopic Examination of the Interface Region in 6061-Al/SiC Composites Reinforced with As-Received and Oxidized SiC Particles. Metall. Trans. A 1990, 21, 2489–2496. [Google Scholar] [CrossRef]
- Van Trinh, P.; Lee, J.; Minh, P.N.; Phuong, D.D.; Hong, S.H. Effect of oxidation of SiC particles on mechanical properties and wear behavior of SiCp/Al6061 composites. J. Alloy. Compd. 2018, 769, 282–292. [Google Scholar] [CrossRef]







| Materials | Tensile Strength (MPa) | Uniform Elongation (%) |
|---|---|---|
| Ag-SnO2 composite | 296 ± 6 | 5 ± 1 |
| Ag-La2Sn2O7 composite | 243 ± 19 | 19 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Cai, W.; Bao, N.; Geng, X.; Yang, H. Superior Plasticity of Silver-Based Composites with Reinforcing Pyrochlore. Metals 2023, 13, 325. https://doi.org/10.3390/met13020325
Zhang L, Cai W, Bao N, Geng X, Yang H. Superior Plasticity of Silver-Based Composites with Reinforcing Pyrochlore. Metals. 2023; 13(2):325. https://doi.org/10.3390/met13020325
Chicago/Turabian StyleZhang, Lingjie, Weiwei Cai, Ningzhong Bao, Xueyu Geng, and Hui Yang. 2023. "Superior Plasticity of Silver-Based Composites with Reinforcing Pyrochlore" Metals 13, no. 2: 325. https://doi.org/10.3390/met13020325