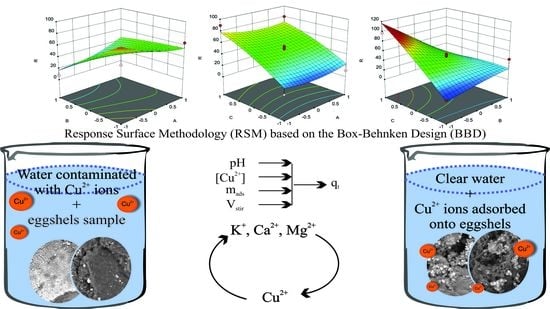
Raw Eggshell as an Adsorbent for Copper Ions Biosorption—Equilibrium, Kinetic, Thermodynamic and Process Optimization Studies
Abstract
:1. Introduction
- –
- the influence of different process parameters (initial Cu2+ ions concentration, pH value of the solution, adsorbent mass and stirring rate) on the biosorption capacity;
- –
- SEM-EDS analysis of the eggshells sample before and after the biosorption process;
- –
- kinetic analysis of the biosorption process;
- –
- equilibrium analysis of the biosorption process;
- –
- thermodynamic analysis of the biosorption process;
- –
- process optimization study by the mean of Response Surface Methodology based on Box-Behnken Design
2. Materials and Methods
3. Results and Discussions
3.1. The Influence of Different Process Parameters on the Adsorption Efficiency (Biosorption Capacity)
3.1.1. The Effect of ph Value on the Biosorption Capacity
3.1.2. The Influence of the Initial Cu2+ Concentration on the Biosorption Capacity
3.1.3. The Effect of the Adsorbent Mass on the Biosorption Capacity
3.1.4. The Influence of the Stirring Rate on the Biosorption Capacity
3.2. SEM-EDS Analysis
3.3. Kinetic Study
3.3.1. Pseudo-First Order Kinetic Model
3.3.2. Pseudo-Second Order Kinetic Model
3.3.3. Intraparticle Diffusion Kinetic Model (Weber-Morris Model)
3.3.4. Elovich Kinetic Model
3.4. Equilibrium Study
3.4.1. Langmuir Isotherm Model
3.4.2. Freundlich Isotherm Model
3.4.3. Temkin Isotherm Model
3.5. Thermodynamic Study
3.6. Process Optimization Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xin, Y.; Li, C.; Liu, J.; Liu, J.; Liu, Y.; He, W.; Gao, Y. Adsorption of heavy metal with modified eggshell membrane and the in situ synthesis of Cu-Ag/modified eggshell membrane composites. R. Soc. Open Sci. 2018, 5, 180532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Dong, X.; Mu, X.; Wang, Y.; Chen, J. Constructing adjacent phosphine oxide ligands confined in mesoporous Zr-MOFs for uranium capture from acidic medium. J. Mater. Chem. A 2021, 9, 16685. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of heavy metal ions from wastewater: A comprehensive and critical review. Npj Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Long, M.; Jiang, H.; Li, X. Biosorption of Cu2+, Pb2+, Cd2+ and their mixture from aqueous solutions by Michelia figo sawdust. Sci. Rep. 2021, 11, 11527. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Joshi, U.M. Chicken eggshells remove Pb(II) ions from synthetic wastewater. Environ. Eng. Sci. 2013, 30, 67–73. [Google Scholar] [CrossRef]
- Cordeiro, C.M.M.; Hincke, M.T. Recent patents on eggshell: Shell and membrane applications. Recent Pat. Food Nutr. Agric. 2011, 3, 1–8. [Google Scholar] [CrossRef]
- Su, W.; Yang, Y.; Dai, H.; Jiang, L. Biosorption of heavy metal ions from aqueous solution o Chinese fir bark modified by sodium hypochlorite. Bioresources 2015, 10, 6993–7008. [Google Scholar] [CrossRef] [Green Version]
- Imessaoudene, A.; Cheikh, S.; Bollinger, J.C.; Belkhiri, L.; Tiri, A.; Bouzaza, A.; El Jery, A.; Assadi, A.; Amrane, A.; Mouni, L. Zeolite waste characterization and use as low-cost, ecofriendly, and sustainable material for malachite green and methylene blue dyes removal: Box-behnken design, kinetics and thermodynamics. Appl. Sci. 2022, 12, 7587. [Google Scholar] [CrossRef]
- Murithi, G.; Onindo, C.O.; Muthakia, G.K. Kinetic and equilibrium study for the sorption of Pb(II) ions from aqueous phase by water hyacinth (Eichhornia crassipes). Bull. Chem. Soc. Ethiop. 2012, 26, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Tong, X.; Deng, Z.; Lv, X. The adsorption of Cu species onto pyrite surface and its effect on pyrite flotation. J. Chem. 2016, 2016, 4627929. [Google Scholar] [CrossRef]
- Putra, W.P.; Kamari, A.; Yusoff, S.N.M.; Ishak, C.F.; Mohamed, A.; Norhayati, H.; Isa, I.M. Biosorption of Cu (II), Pb (II) and Zn (II) ions from aqueous solutions using selected waste materials: Adsorption and characterization studies. JEAS 2014, 4, 43523. [Google Scholar] [CrossRef] [Green Version]
- Anantha, R.K.; Kota, S. An evaluation of the major factors influencing the removal of copper ions using the egg shell (Dromaius novaehollandiae): Chitosan (Agaricus bisporus) composite. Biotech 2016, 6, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Sven. Vetenskapsakad. Handingarl. 1898, 241, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Cheung, W.H.; Szeto, Y.S.; McKay, G. Intraparticle diffusion processes during acid dye adsorption onto chitosan. Bioresour. Technol. 2007, 98, 2897–2904. [Google Scholar] [CrossRef] [PubMed]
- Elkady, M.F.; Ibrahim, A.M.; El Latif, M.M.A. Assessment of the adsorption kinetics, equilibrium, and thermodynamic for the potential removal of reactive red dye using eggshel biocomposite beads. Desalination 2011, 278, 412–423. [Google Scholar] [CrossRef]
- Gorgievski, M.; Božić, D.; Stanković, V.; Štrbac, N.; Šerbula, S. Kinetics, equilibrium and mechanism of Cu2+, Ni2+ and Zn2+ ions biosorption using wheat straw. Ecol. Eng. 2013, 58, 113–122. [Google Scholar] [CrossRef]
- Tonk, S.; Majdik, C.; Szep, R.; Suciu, M.; Rapo, E.; Nagy, B.; Niculae, A.G. Biosorption of Cd(II) ions from aqueous solution onto eggshell waste, kinetic and equilibrium isotherm studies. Rev. Chim. 2017, 68, 1951–1958. [Google Scholar] [CrossRef]
- Bouchelkia, N.; Mouni, L.; Belkhiri, L.; Bouzaza, A.; Bollinger, J.C.; Madani, K.; Dahmoun, F. Removal of lead(II) from water using activated carbon developed from jujube stones, a low cost adsorbent. Sep. Sci. Technol. 2016, 51, 1645–1653. [Google Scholar] [CrossRef]
- Metwally, S.S.; Rizk, H.E.; Gasser, M.S. Biosorption of strontium ions from aqueous solution using modified eggshell materials. Radiochim. Acta 2017, 105, 1021–1031. [Google Scholar] [CrossRef]
- Saha, P.D.; Chowdhury, S.; Mondal, M.; Sinha, K. Biosorption of direct red 28 (congo red) from aqueous solutions by eggshells: Batch and column studies. Sep. Sci. Technol. 2014, 47, 112–123. [Google Scholar] [CrossRef]
- Savastru, E.; Bulgariu, D.; Zamfir, C.I.; Bulgariu, L. Application of Saccharomyces cerevisiae in the biosorption of Co (II), Zn (II), and Cu (II) ions from aqueous media. Water 2022, 14, 976. [Google Scholar] [CrossRef]
- Sireesha, C.; Subha, R.; Sumithra, S. Biosorption of copper ions in aqueous solution by carbonized sunflower stem. Rasayan J. Chem. 2022, 15, 2267–2273. [Google Scholar] [CrossRef]
- Marques, G.S.; Dusi, G.G.; Drago, F.; Gimenes, M.L.; da Silva, V.R. Biosorption of Cu(II) ions using sericin cross-linked with polyethylene glycol-diglycidyl ether. Desalination Water Treat. 2022, 270, 153–162. [Google Scholar] [CrossRef]
- Božić, D.; Stanković, V.; Gorgievski, M.; Bogdanović, G.; Kovačević, R. Adsorption of heavy metal ions by sawdust of deciduous trees. J. Hazard. Mater. 2009, 171, 684–692. [Google Scholar] [CrossRef]
- Moreira, V.R.; Lebron, Y.A.R.; Freire, S.J.; Santos, L.V.S.; Palladino, F.; Jacob, R.S. Biosorption of copper ions from aqueous solution using Chlorella pyrenoidosa: Optimization, equilibrium and kinetic studies. Microchem. J. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Fawzy, M.A. Biosorption of copper ions from aqueous solution by Codium vermilara: Optimization, kinetic, isotherm and thermodynamic studies. Adv. Powder. Technol. 2020, 31, 3724–3735. [Google Scholar] [CrossRef]
- Blazquez, G.; Martin-Lara, M.A.; Dionsio-Ruiz, E.; Tenorio, G.; Calero, M. Evaluation and comparison of the biosorption process of copper ions onto olive stone and pine bark. J. Ind. Eng. Chem. 2011, 17, 824–833. [Google Scholar] [CrossRef]
- Modrzejewska, Z.; Rogacki, G.; Sujka, W.; Zarzycki, R. Sorption of copper by chitosan hydrogel: Kinetics and equilibrium. Chem. Eng. Process. 2016, 109, 104–113. [Google Scholar] [CrossRef]
- Awwad, K.M.; Farhan, A.M. Equilibrium, kinetic and thermodynamics of biosorption of lead (II) copper (II) and cadmium (II) ions from aqueous solutions onto olive leaves powder. Am. J. Chem. 2012, 2, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Thilagan, J.; Gopalakrishnan, S.; Kannadasan, T. Thermodynamic study on adsorption of copper (II) ions in aqueous solution by chitosan blended with cellulose & cross linked by formaldehyde, chitosan immobilised on red soil, chitosan reinforced by banana stem fibre. IJSRET 2013, 2, 28–36. [Google Scholar]
- Ozel, H.U.; Gemici, B.T.; Ozel, H.B.; Berberler, E. Evaluating forest waste on adsorption of Cd(II) from aqueous solution: Equilibrium and thermodynamic studies. Pol. J. Environ. Stud. 2019, 28, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Choinska-Pulit, A.; Sobolczyk-Bednarek, J.; Laba, W. Optimization of copper, lead and cadmium biosorption onto newly isolated bacterium using a Box-Behnken design. Ecotoxicol. Environ. Saf. 2018, 149, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Mammar, A.C.; Mouni, L.; Bollinger, J.C.; Belkhiri, L.; Bouzaza, L.; Bouzaza, A.; Assadi, A.A.; Belkacemi, H. Modeling and optimization of process parameters in elucidating the adsorption mechanism of gallic acid on activated carbon prepared from date stones. Sep. Sci. Technol. 2020, 55, 3113–3125. [Google Scholar] [CrossRef]
- Turkyilmaz, H.; Kartal, T.; Yigitarslan Yildiz, S. Optimization of lead adsorption of mordenite by response surface methodology: Characterization and modification. J. Environ. Health Sci. Eng. 2014, 12, 5. [Google Scholar] [CrossRef]
- Design-Expert® Software, version 22.0.0; Stat-Ease, Inc.: Minneapolis, MN, USA, 2022; Available online: https://www.statease.com (accessed on 22 December 2022).











| Model | Parameters | Values |
|---|---|---|
| Pseudo-first order kinetic model | k1 (min−1) | 0.018 |
| qe,exp (mg g−1) | 22.84 | |
| qe,cal (mg g−1) | 28.34 | |
| R2 | 0.999 | |
| Pseudo-second order kinetic model | k2 (g mg−1 min−1) | 2.90∙10−4 |
| qe,exp (mg g−1) | 22.84 | |
| qe,cal (mg g−1) | 43.26 | |
| R2 | 0.982 | |
| Intraparticle diffusion kinetic model (Weber-Morris model) | Ki (mg g−1 min−0.5) | 2.311 |
| Ci | 9.99·10−24 | |
| R2 | 0.955 | |
| Elovich kinetic model | α (mg g−1 min−1) | 0.603 |
| β (g mg−1) | 0.067 | |
| R2 | 0.983 |
| Model | Parameters | Values |
|---|---|---|
| Langmuir adsorption isotherm model | KL (dm3 mg−1) | 3.49 |
| qe,exp (mg g−1) | 28.3 | |
| qm (mg g−1) | 94.59 | |
| R2 | 0.989 | |
| Freundlich adsorption isotherm model | KF | 108.5 |
| 1/n | 0.671 | |
| R2 | 0.931 | |
| Temkin adsorption isotherm model | B (J mol−1) | 9.698 |
| KT (dm3 g−1) | 104.49 | |
| R2 | 0.927 |
| Biosorbent | Maximum Biosorbent Capacity (qm, mg g−1) | Work |
|---|---|---|
| Eggshell | 94.59 | This work |
| Saccharomyces cerevisiae (brewer’s yeast) | 26.95 | [22] |
| Carbonized sunflower stem | 38.05 | [23] |
| Sericin cross-linked with polyethylene glycol-diglycidyl ether | 36.17 | [24] |
| Sawdust of deciduous trees | 9.9 | [25] |
| Wheat straw | 4.3 | [17] |
| Chlorella pyrenoidosa (freshwater green algae) | 11.88 | [26] |
| Codium vermilara (codium seaweed) | 14.4 | [27] |
| Olive stone | 1.96 | [28] |
| Pine bark | 11.35 | [28] |
| Chitosan | 103 | [29] |
| T (K) | ΔG0 (kJ mol −1) | ΔH0 (kJ mol −1) | ΔS0 (J mol −1 K−1) | Ea (kJ mol −1) |
|---|---|---|---|---|
| 298 | 0.07 | −9.98 | 33.68 | 82.97 |
| 308 | −4.51 | |||
| 318 | −5.45 |
| Factors | Range Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A—Adsorbent mass (g) | 0.5 | 1 | 1.5 |
| B—Initial metal ion concentration (g/L) | 0.5 | 1 | 1.5 |
| C—Contact time (min) | 10 | 60 | 90 |
| Run | A: Adsorbent Mass (g) | B: Initial Cu2+ Ions Concentration (g/L) | C: Contact Time (min) | R: Adsorption Degree (%) |
|---|---|---|---|---|
| 1 | 1.5 | 0.5 | 60 | 64.54 |
| 2 | 0.5 | 1 | 10 | 9.87 |
| 3 | 1 | 1.5 | 10 | 43.09 |
| 4 | 1 | 0.5 | 10 | 7.02 |
| 5 | 1.5 | 1 | 10 | 11.03 |
| 6 | 0.5 | 1.5 | 90 | 12.6 |
| 7 | 0.5 | 1 | 60 | 25.86 |
| 8 | 1.5 | 1 | 90 | 80.47 |
| 9 | 0.5 | 1.5 | 60 | 7.47 |
| 10 | 1 | 1 | 60 | 54.27 |
| 11 | 0.5 | 0.5 | 60 | 96.16 |
| 12 | 1 | 1 | 60 | 51.14 |
| 13 | 1 | 1 | 60 | 52.16 |
| 14 | 1 | 1 | 60 | 49.67 |
| 15 | 1.5 | 1.5 | 60 | 38 |
| 16 | 1 | 0.5 | 90 | 97.06 |
| 17 | 0.5 | 1 | 90 | 89.74 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 13,714.86 | 9 | 1523.87 | 5.03 | 0.0224 | Significant |
| A-A | 10.58 | 1 | 10.58 | 0.0349 | 0.8571 | |
| B-B | 3346.44 | 1 | 3346.44 | 11.04 | 0.0127 | |
| C-C | 5452.81 | 1 | 5452.81 | 17.99 | 0.0038 | |
| AB | 965.66 | 1 | 965.66 | 3.19 | 0.1174 | |
| AC | 27.20 | 1 | 27.20 | 0.0897 | 0.7732 | |
| BC | 3631.87 | 1 | 3631.87 | 11.98 | 0.0105 | |
| A² | 171.32 | 1 | 171.32 | 0.5652 | 0.4767 | |
| B² | 8.93 | 1 | 8.93 | 0.0295 | 0.8686 | |
| C² | 114.79 | 1 | 114.79 | 0.3787 | 0.5578 | |
| Residual | 2121.64 | 7 | 303.09 | |||
| Lack of Fit | 1571.72 | 3 | 523.91 | 3.81 | 0.1145 | Not significant |
| Pure Error | 549.92 | 4 | 137.48 | |||
| Cor Total | 15,836.50 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, M.; Gorgievski, M.; Štrbac, N.; Grekulović, V.; Božinović, K.; Zdravković, M.; Vuković, M. Raw Eggshell as an Adsorbent for Copper Ions Biosorption—Equilibrium, Kinetic, Thermodynamic and Process Optimization Studies. Metals 2023, 13, 206. https://doi.org/10.3390/met13020206
Marković M, Gorgievski M, Štrbac N, Grekulović V, Božinović K, Zdravković M, Vuković M. Raw Eggshell as an Adsorbent for Copper Ions Biosorption—Equilibrium, Kinetic, Thermodynamic and Process Optimization Studies. Metals. 2023; 13(2):206. https://doi.org/10.3390/met13020206
Chicago/Turabian StyleMarković, Miljan, Milan Gorgievski, Nada Štrbac, Vesna Grekulović, Kristina Božinović, Milica Zdravković, and Milovan Vuković. 2023. "Raw Eggshell as an Adsorbent for Copper Ions Biosorption—Equilibrium, Kinetic, Thermodynamic and Process Optimization Studies" Metals 13, no. 2: 206. https://doi.org/10.3390/met13020206