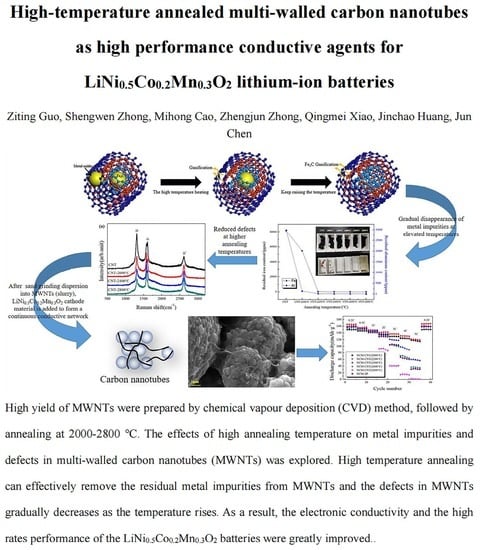
High-Temperature-Annealed Multi-Walled Carbon Nanotubes as High-Performance Conductive Agents for LiNi0.5Co0.2Mn0.3O2 Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Multi-Walled Carbon Nanotubes
2.2. High-Temperature-Annealed Multi-Walled Carbon Nanotubes
2.3. Preparation of Multi-Walled Carbon Nanotube Conductive Slurry
2.4. Assemble of Batteries Using Multi-Walled Carbon Nanotube Slurry as Conductive Agent
2.5. Characterization and Performance Testing
3. Result and Discussion
3.1. Inductively Coupled Plasma Emission Spectroscopy (ICP) Analysis
3.2. X-ray Diffraction (XRD) Analysis
3.3. Raman Spectroscopy
3.4. Scanning Electron Microscopy (SEM) Analysis
3.5. Research in Transmission Electron Microscopy
3.6. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Nykvist, B.; Nilsson, M. Rapidly falling costs of battery packs for electric vehicles. Nat. Clim. Change 2015, 5, 329–332. [Google Scholar] [CrossRef]
- Dudney, N.J.; Li, J. Using all energy in a battery. Science 2015, 347, 131–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Kucinskis, G.; Bajars, G.; Kleperis, J. Graphene in lithium ion battery cathode materials: A review. J. Power Sources 2013, 240, 66–79. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, C.S.; Hwang, J.Y.; Kim, S.J.; Maglia, F.; Lamp, P.; Myung, S.-T.; Sun, Y.K. High-energy-density lithium-ion battery using a carbon-nanotube-Si composite anode and a compositionally graded Li[Ni0.85Co0.05Mn0.1]O2 cathode. Energy Environ. Sci. 2016, 96, 2152–2158. [Google Scholar] [CrossRef]
- Sehrawat, P.; Julien, C.; Islam, S.S. Carbon nanotubes in Li-ion batteries: A review. Mater. Sci. Eng. B 2016, 213, 12–40. [Google Scholar] [CrossRef]
- Chen, Y.T.; Zhang, H.Y.; Chen, T.M. Graphene-carbon nano-tubes-modified LiFePO4 cathode materials for high-performance lithium-ion batteries. Mater. Sci. Forum 2018, 913, 810–830. [Google Scholar]
- Wu, X.L.; Guo, Y.G.; Su, J. Carbon-Nanotube-Decorated Nano-LiFePO4 @C Cathode Material with Superior High-Rate and Low-Temperature Performances for Lithium-Ion Batteries. Adv. Energy Mater. 2013, 3, 1155–1160. [Google Scholar] [CrossRef]
- Wang, B.; Liu, T.F.; Liu, A.M.; Liu, G.J. A hierarchical porous C@LiFePO4/carbon nanotubes microsphere composite for high-rate lithium-ion batteries: Combined experimental and theoretical study. Adv. Energy Mater. 2016, 6, 1600426–1600435. [Google Scholar] [CrossRef]
- Liu, X.Y.; Peng, H.J.; Zhang, Q.; Huang, J.Q.; Liu, X.F.; Wang, L.; He, X.M.; Zhu, W.C.; Wei, F. Hierarchical carbon nanotube/carbon black scaffolds as short-and long-range electron pathways with superior Li-ion storage performance. ACS Sustain. Chem. Eng. 2013, 2, 200–206. [Google Scholar] [CrossRef]
- Chung, D.D.L. Carbon Fiber Composite; Butterworth-Heinemann: Boston, MA, USA, 1994. [Google Scholar]
- Murdie, N. Introduction to Carbon Technologies; Marsh, H., Heintz, E.A., Rodriguez-Reinoso, F., Eds.; University of Alicante: Alicante, Spain, 1997; pp. 597–634. [Google Scholar]
- Lambert, J.M.; Ajayan, P.M.; Bernier, P.; Planeix, J.M. Improving conditions towards isolating single-shell carbon nanotubes. Chem. Phys. Lett. 1994, 226, 364–371. [Google Scholar] [CrossRef]
- Andrews, R.; Jacques, D.; Qian, D.; Dickey, E.C. Purification and structural annealing of multiwalled carbon nanotubes at graphitization temperatures. Carbon 2001, 39, 1681–1687. [Google Scholar] [CrossRef]
- Rennhofer, H.; Zanghellini, B. Dispersion state and damage of carbon nantubes and carbon nanofibers by ultrasonic dispersion: A Review. Nanomaterials 2021, 11, 1469. [Google Scholar] [CrossRef]
- Park, S.; Choi, S.-W.; Jin, C. Dispersion of multi-walled carbon nanotubes mechanically milled under different process conditions. Mater. Chem. Phys. 2019, 236, 121798. [Google Scholar] [CrossRef]
- Tarawneh, M.; Ahmad, S.; EhNoum, S.Y.; Lau, K.-T. Sonication effect on the mechanical properties of MWCNTs reinforced natural rubber. J. Compos. Mater. 2012, 47, 579–585. [Google Scholar] [CrossRef]
- Woo, J.S.; Bang, D.-S.; Lee, G.-W.; Kye, H.S.; Shin, K.C. Characterization of PMMA/MWNT Composites Fabricated by a Twin Screw Extruder. Elastomers Compos. 2007, 42, 151–158. [Google Scholar]
- Ding, W.; Eitan, A.; Fisher, F.T.; Chen, X.; Dikin, D.A.; Andrews, R.; Brinson, L.C.; Schadler, L.S.; Ruoff, R.S. Direct Observation of Polymer Sheathing in Carbon Nanotube−Polycarbonate Composites. Nano Lett. 2003, 3, 1593–1597. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef]
- Kim, Y.A.; Hayashi, T.; Osawa, K.; Dresselhaus, M.S.; Endo, M. Annealing effect on disordered multi-wall carbon nanotubes. Chem. Phys. Lett. 2003, 380, 319–324. [Google Scholar] [CrossRef]
- Hou, P.X.; Liu, C.; Cheng, H.M. Purification of carbon nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Kuznetsov, V.L.; Elumeeva, K.V.; Ishchenko, A.V.; Beylina, N.Y.; Stepashkin, A.A.; Moseenkov, S.I.; Plyasova, L.M.; Molina, I.Y.; Romanenko, A.I.; Anikeeva, O.B. Multi-walled carbon nanotubes with ppm level of impurities. Phys. Status Solidi Basic Res. 2010, 247, 2695–2699. [Google Scholar] [CrossRef]
- Saito, Y.; Yoshikawa, T.; Bandow, S.; Tomita, M.; Hayashi, T. Interlayer spacings in carbon nanotubes. Phys. Rev. B 1993, 48, 1907–1909. [Google Scholar] [CrossRef]
- Lian, Y.; Zheng, Y.; Wang, Z.; Hu, Y.; Zhao, J.; Zhang, H. Multidefect N-Nb2O5-x@CNTs Incorporated into Capillary Transport Framework for Li+/Na+ Storage. Small 2022, 18, 2201450. [Google Scholar] [CrossRef]
- Shimoda, H.; Gao, B.; Tang, X.P.; Kleinhammes, A.; Fleming, L.; Wu, Y.; Zhou, O. Lithium Intercalation into Opened Single-Wall Carbon Nanotubes: Storage Capacity and Electronic Properties. Phys. Rev. Lett. 2022, 88, 015502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houska, C.R.; Warren, B.E. X-ray study of the graphitization of carbon black. J. Appl. Phys. 1954, 25, 1503. [Google Scholar] [CrossRef]
- Boloyov, V.V.; Knyazev, E.V.; Korusenko, P.M.; Nesov, S.N.; Sachkov, V.A. Functionalization of individual multi-wall carbon nanotubes during irradiation and annealing. Phys. Solid State 2020, 62, 2173–2183. [Google Scholar] [CrossRef]
- Frackowial, E.; Gauiter, S.; Gaucher, H. Electrochemical storage of lithium multiwalled carbon nanotubes. Carbon 1999, 37, 61–69. [Google Scholar] [CrossRef]
- Wen, L.; Wang, L.; Guan, Z.; Liu, X.; Wei, M.; Jiang, D.; Zhang, S. Effect of composite conductive agent on internal resistance and performance of lithium iron phosphate batteries. Ionics 2022, 28, 3145–3153. [Google Scholar] [CrossRef]








| Sample Number (ppm) | Fe | Al |
|---|---|---|
| CNT | 81700 | 29800 |
| CNT-2000 ℃ | 56400 | 1400 |
| CNT-2200 ℃ | 300 | 189 |
| CNT-2400 ℃ | 150 | 132 |
| CNT-2600 ℃ | 118 | 125 |
| CNT-2800 ℃ | <100 | <100 |
| Sample | R1 (mΩ) | R2 (mΩ) |
|---|---|---|
| NCM-CNT (2000 ℃) | 6.972 | 190.6 |
| NCM-CNT (2200 ℃) | 1.142 | 107.1 |
| NCM-CNT (2400 ℃) | 0.261 | 129.6 |
| NCM-CNT (2600 ℃) | 2.007 | 364.3 |
| NCM-CNT (2800 ℃) | 6.571 | 298.9 |
| NCM-SP NCM-CNT | 4.368 3.552 | 271.7 707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Z.; Zhong, S.; Cao, M.; Zhong, Z.; Xiao, Q.; Huang, J.; Chen, J. High-Temperature-Annealed Multi-Walled Carbon Nanotubes as High-Performance Conductive Agents for LiNi0.5Co0.2Mn0.3O2 Lithium-Ion Batteries. Metals 2023, 13, 36. https://doi.org/10.3390/met13010036
Guo Z, Zhong S, Cao M, Zhong Z, Xiao Q, Huang J, Chen J. High-Temperature-Annealed Multi-Walled Carbon Nanotubes as High-Performance Conductive Agents for LiNi0.5Co0.2Mn0.3O2 Lithium-Ion Batteries. Metals. 2023; 13(1):36. https://doi.org/10.3390/met13010036
Chicago/Turabian StyleGuo, Ziting, Shengwen Zhong, Mihong Cao, Zhengjun Zhong, Qingmei Xiao, Jinchao Huang, and Jun Chen. 2023. "High-Temperature-Annealed Multi-Walled Carbon Nanotubes as High-Performance Conductive Agents for LiNi0.5Co0.2Mn0.3O2 Lithium-Ion Batteries" Metals 13, no. 1: 36. https://doi.org/10.3390/met13010036