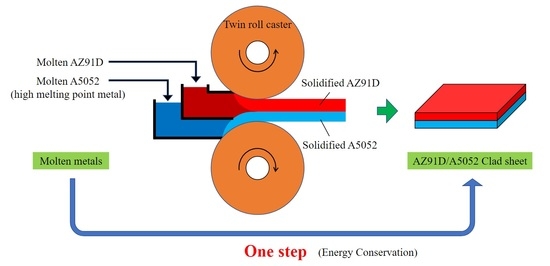
Fabrication of Mg/Al Clad Strips by Direct Cladding from Molten Metals
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials and Methods
2.2. Control of Clad Ratio
2.3. Cladding for Mg/Al Clad Strips by Twin-Roll Caster
2.4. Microstructure of the Bonding Interface
3. Results and Discussion
3.1. Calculation of the Experimental Solidification Constants
3.2. Effects of Roll Speeds and Pouring Sequences on the Surfaces of the Clad Strips
3.3. Microstructure of the Bonding Interface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taub, A.I.; Luo, A.A. Advanced lightweight materials and manufacturing processes for automotive applications. MRS Bull. 2015, 40, 1045–1054. [Google Scholar] [CrossRef]
- Hovorun, T.P.; Berladir, K.V.; Pererva, V.I.; Rudenko, S.G.; Martynov, A.I. Modern materials for automotive industry. J. Eng. Sci. 2017, 4, f8–f18. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Ponder, K.; Brizes, E.; Abke, T.; Edwards, P.; Ramirez, A.J. Combining resistance spot welding and friction element welding for dissimilar joining of aluminum to high strength steels. J. Mater. Process. Technol. 2019, 273, 116192. [Google Scholar] [CrossRef]
- Yang, J.; Oliveira, J.P.; Li, Y.; Tan, C.; Gao, C.; Zhao, Y.; Yu, Z. Laser techniques for dissimilar joining of aluminum alloys to steels: A critical review. J. Mater. Process. Technol. 2022, 301, 117443. [Google Scholar] [CrossRef]
- Cole, G.S.; Sherman, A.M. Light weight materials for automotive applications. Mater. Charact. 1995, 35, 3–9. [Google Scholar] [CrossRef]
- Khorasani, M.; Ghasemi, A.; Leary, M.; Sharabian, E.; Cordova, L.; Gibson, I.; Downing, D.; Bateman, S.; Brandt, M.; Rolfe, B. The effect of absorption ratio on meltpool features in laser-based powder bed fusion of IN718. Opt. Laser Technol. 2022, 153, 108263. [Google Scholar] [CrossRef]
- Linares, J.; Chaves-Jacob, J.; Lopez, Q.; Sprauel, J.-M. Fatigue life optimization for 17-4Ph steel produced by selective laser melting. Rapid Prototyp. J. 2022, 28, 1182–1192. [Google Scholar] [CrossRef]
- Giganto, S.; Martínez-Pellitero, S.; Cuesta, E.; Zapico, P.; Barreiro, J. Proposal of design rules for improving the accuracy of selective laser melting (SLM) manufacturing using benchmarks parts. Rapid Prototyp. J. 2022, 28, 1129–1143. [Google Scholar] [CrossRef]
- Khan, H.M.; Waqar, S.; Koç, E. Evolution of temperature and residual stress behavior in selective laser melting of 316L stainless steel across a cooling channel. Rapid Prototyp. J. 2022, 28, 1272–1283. [Google Scholar] [CrossRef]
- Paul, H.; Chulist, R.; Mania, I. Structural Properties of Interfacial Layers in Tantalum to Stainless Steel Clad with Copper Interlayer Produced by Explosive Welding. Metals 2020, 10, 969. [Google Scholar] [CrossRef]
- Kang, M.; Zhou, L.; Deng, Y.; Luo, Y.; He, M.; Zhang, N.; Huang, Z.; Dong, L. Microstructure and Mechanical Properties of 4343/3003/6111/3003 Four-Layer Al Clad Sheets Subjected to Different Conditions. Metals 2022, 12, 777. [Google Scholar] [CrossRef]
- Murzin, S.P.; Palkowski, H.; Melnikov, A.A.; Blokhin, M.V. Laser Welding of Metal-Polymer-Metal Sandwich Panels. Metals 2022, 12, 256. [Google Scholar] [CrossRef]
- Xu, J.; Fu, J.; Li, S.; Xu, G.; Li, Y.; Wang, Z. Effect of annealing and cold rolling on interface microstructure and properties of Ti/Al/Cu clad sheet fabricated by horizontal twin-roll casting. J. Mater. Res. Technol. 2022, 16, 530–543. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, C.; Yang, Y.; Wang, Y.; Sheng, L.; Li, Y.; Huo, M.; Zhang, K.; Xing, L.; Zhang, G. Study on the Microstructure and Mechanical Properties of a Ti/Mg Alloy Clad Plate Produced by Explosive Welding. Metals 2022, 12, 399. [Google Scholar] [CrossRef]
- Song, J.; She, J.; Chen, D.; Pan, F. Latest research advances on magnesium and magnesium alloys worldwide. J. Magnes. Alloy. 2020, 8, 1–41. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.; Cao, X.; Wu, J. The effect of annealing on the interface microstructure and mechanical characteristics of AZ31B/AA6061 composite plates fabricated by explosive welding. Mater. Des. 2015, 65, 1100–1109. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Liu, P.; Geng, H. Microstructure and XRD analysis in the interface zone of Mg/Al diffusion bonding. J. Mater. Process. Technol. 2008, 205, 146–150. [Google Scholar] [CrossRef]
- Jin, H.; Javaid, A. A new cladding technology to bond aluminium on magnesium. Mater. Sci. Technol. 2020, 36, 1037–1043. [Google Scholar] [CrossRef]
- Matsumoto, H.; Watanabe, S.; Hanada, S. Fabrication of pure Al/Mg–Li alloy clad plate and its mechanical properties. J. Mater. Process. Technol. 2005, 169, 9–15. [Google Scholar] [CrossRef]
- Kim, J.-S.; Lee, K.S.; Kwon, Y.N.; Lee, B.-J.; Chang, Y.W.; Lee, S. Improvement of interfacial bonding strength in roll-bonded Mg/Al clad sheets through annealing and secondary rolling process. Mater. Sci. Eng. A 2015, 628, 1–10. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, W.; Li, H. Effect of thickness of interfacial intermetallic compound layers on the interfacial bond strength and the uniaxial tensile behaviour of 5052 Al/AZ31B Mg/5052 Al clad sheets. RSC Adv. 2015, 5, 104954–104959. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Z.; Hu, C.; Li, B.; Mo, T.; Liu, Q. Effects of annealing on the interfacial structures and mechanical properties of hot roll bonded Al/Mg clad sheets. Mater. Sci. Eng. A 2020, 792, 139673. [Google Scholar] [CrossRef]
- Li, S.; Liu, X.; Jia, Y.; Han, J.; Wang, T. Interface Characteristics and Bonding Performance of the Corrugated Mg/Al Clad Plate. Materials 2021, 14, 4412. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Li, Y.; Cao, X.; Peng, R.; Fang, J. Effect of secondary rolling on the interfacial bonding strength and mechanical properties of Al/Mg/Al clad plates. Philos. Mag. Lett. 2022, 102, 200–208. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, Y.; Li, X.; Li, X.; Zhao, T. Effect of inert gas-shielding on the interface and mechanical properties of Mg/Al explosive welding composite plate. J. Manuf. Process. 2019, 45, 166–175. [Google Scholar] [CrossRef]
- Inao, D.; Mori, A.; Tanaka, S.; Hokamoto, K. Explosive Welding of Thin Aluminum Plate onto Magnesium Alloy Plate Using a Gelatin Layer as a Pressure-Transmitting Medium. Metals 2020, 10, 106. [Google Scholar] [CrossRef]
- Rouzbeh, A.; Sedighi, M.; Hashemi, R. Comparison between Explosive Welding and Roll-Bonding Processes of AA1050/Mg AZ31B Bilayer Composite Sheets Considering Microstructure and Mechanical Properties. J. Mater. Eng. Perform. 2020, 29, 6322–6332. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Shi, B.; Wu, Y. Experimental and Numerical Studies on Preparation of Thin AZ31B/AA5052 Composite Plates Using Improved Explosive Welding Technique. Metals 2020, 10, 1023. [Google Scholar] [CrossRef]
- Atifeh, S.M.; Rouzbeh, A.; Hashemi, R.; Sedighi, M. Effect of annealing on formability and mechanical properties of AA1050/Mg-AZ31B bilayer sheets fabricated by explosive welding method. Int. J. Adv. Manuf. Technol. 2022, 118, 775–784. [Google Scholar] [CrossRef]
- Bae, J.H.; Prasada Rao, A.K.; Kim, K.H.; Kim, N.J. Cladding of Mg alloy with Al by twin-roll casting. Scr. Mater. 2011, 64, 836–839. [Google Scholar] [CrossRef]
- Park, J.; Song, H.; Kim, J.-S.; Sohn, S.S.; Lee, S. Three-Ply Al/Mg/Al Clad Sheets Fabricated by Twin-Roll Casting and Post-treatments (Homogenization, Warm Rolling, and Annealing). Metall. Mater. Trans. A 2017, 48, 57–62. [Google Scholar] [CrossRef]
- Haga, T.; Nakamura, R.; Kumai, S.; Watari, H. A vertical type twin roll caster for an aluminium alloy clad strip. Arch. Mater. Sci. Eng. 2013, 62, 36–44. [Google Scholar]
- Haga, T. Twin Roll Caster for Clad Strip. Metals 2021, 11, 776. [Google Scholar] [CrossRef]
- Feng, G.Y.; Watari, H.; Suzuki, M.; Haga, T.; Shimizu, T. Novel Direct Cladding of Magnesium and Aluminum Alloys Using a Horizontal Twin Roll Caster. Key Eng. Mater. 2021, 880, 17–22. [Google Scholar] [CrossRef]
- Haga, T. High Speed Roll Caster for Aluminum Alloy. Metals 2021, 11, 520. [Google Scholar] [CrossRef]
- Liu, N.; Liu, C.; Liang, C.; Zhang, Y. Influence of Ni Interlayer on Microstructure and Mechanical Properties of Mg/Al Bimetallic Castings. Metall. Mater. Trans. A 2018, 49, 3556–3564. [Google Scholar] [CrossRef]
- Li, G.; Jiang, W.; Guan, F.; Zhu, J.; Zhang, Z.; Fan, Z. Microstructure, mechanical properties and corrosion resistance of A356 aluminum/AZ91D magnesium bimetal prepared by a compound casting combined with a novel Ni-Cu composite interlayer. J. Mater. Process. Technol. 2021, 288, 116874. [Google Scholar] [CrossRef]
- Shu, J.; Yamaguchi, T.; Hara, Y. Influence of a Ni Foil Interlayer on Interface Properties of Mg-Clad Al Materials by Vacuum Roll Bonding. Mater. Trans. 2020, 61, 1020–1025. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Z.; Chang, L.; Guo, D.; Yu, J.; Cui, M. A two-step bonding process for preparing 6061/AZ31 bimetal assisted with liquid molten zinc interlayer: The process and microstructure. J. Adhes. Sci. Technol. 2021, 1–23. [Google Scholar] [CrossRef]
- Dong, S.; Lin, S.; Zhu, H.; Wang, C.; Cao, Z. Effect of Ni interlayer on microstructure and mechanical properties of Al/Mg dissimilar friction stir welding joints. Sci. Technol. Weld. Join. 2022, 27, 103–113. [Google Scholar] [CrossRef]










| Materials | Mg | Al | Si | Fe | Cu | Mn | Zn | Cr |
|---|---|---|---|---|---|---|---|---|
| AZ91D | Rest | 9 | 0.05 | 0.002 | 0.01 | 0.3 | 1 | - |
| A5052 | 2.5 | Rest | 0.09 | 0.14 | 0.01 | 0.01 | - | 0.25 |
| Materials | AZ91D | A5052 |
|---|---|---|
| Solidus temperature [°C] | 430 | 607 |
| Liquidus temperature [°C] | 595 | 649 |
| Pouring temperature [°C] | 600 | 654 |
| Solidification length [mm] | 50 | |
| Cooling length [mm] | 50 | |
| Roll speed [m/min] | 6–36 | |
| Materials | AZ91D | A5052 |
|---|---|---|
| Pouring temperature [°C] | 600 | 654 |
| Pouring sequence [upper nozzle/lower nozzle] | A5052/AZ91D, AZ91D/A5052 | |
| Upper solidification length [mm] | 50, 100 | |
| Solidification length [mm] | 50 | |
| Cooling length [mm] | 50 | |
| Roll gap [mm] | 5 | |
| Roll speed [m/min] | 6–12 | |
| Roll surface temperature [°C] | 22 | |
| Pouring Sequence [Upper Nozzle/Lower Nozzle] | Roll Speed [m/min] | ||
|---|---|---|---|
| 6 | 9 | 12 | |
| A5052/AZ91D | × | × | × |
| AZ91D/A5052 | × | ∆ | × |
| Point | Mg | Al | Possible Phase |
|---|---|---|---|
| 1 | 90.15 | 9.49 | α-Mg |
| 2 | 66.70 | 33.30 | α-Mg+Mg17Al12 |
| 3 | 56.86 | 43.14 | Mg17Al12 |
| 4 | 43.32 | 56.68 | Al3Mg2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.; Watari, H.; Haga, T. Fabrication of Mg/Al Clad Strips by Direct Cladding from Molten Metals. Metals 2022, 12, 1408. https://doi.org/10.3390/met12091408
Feng G, Watari H, Haga T. Fabrication of Mg/Al Clad Strips by Direct Cladding from Molten Metals. Metals. 2022; 12(9):1408. https://doi.org/10.3390/met12091408
Chicago/Turabian StyleFeng, Gengyan, Hisaki Watari, and Toshio Haga. 2022. "Fabrication of Mg/Al Clad Strips by Direct Cladding from Molten Metals" Metals 12, no. 9: 1408. https://doi.org/10.3390/met12091408