Effect of Fe2O3 on the Crystallization Behavior of Glass-Ceramics Produced from Secondary Nickel Slag
Abstract
:1. Introduction
2. Experiment
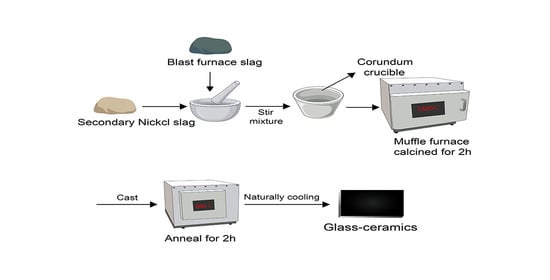
2.1. Glass Preparation
2.2. Characterizations
3. Results and Discussion
3.1. Thermal Behavior of the Base Glass
3.2. Crystalline Phase and Morphological Structures Analysis
3.3. Analysis of the Glass Network Structure
3.4. Physicochemical Properties of Glass-Ceramics
4. Conclusions
- (1)
- With the increase in Fe2O3 content, the crystallization temperature of glass-ceramics first decreases and then increases. Fe2O3 can effectively reduce the crystallization temperature and improve the crystallization capacity.
- (2)
- The crystal phases precipitated are anorthite and ferrobustamite, and crystallinity reaches a maximum of 72.45% with Fe2O3 content of 9.32 wt%. The microstructure of glass-ceramics becomes dense with the increase in Fe2O3.
- (3)
- Fe2O3 changes the value of NBO/T in the glass, increasing first and then decreasing. Excessive Fe2O3 will weaken the depolymerization of the [SiO4] tetrahedral structure, as when Fe2O3 reaches 12.42 wt%.
- (4)
- When the content of Fe2O3 is 9.32 wt%, the Vickers hardness, bending strength, acid resistance and alkali resistance of this sample are 11.42 GPa and 121 MPa, 99.81% and 99.70%. The performances meet the requirements of industrial glass-ceramic plates (JC/T 2097-2011).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deubener, J.; Allix, M.; Davis, M.J. Updated definition of glass-ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Tao, J.; Yang, Z.X. Influence of different activators on microstructure and strength of alkali-activated nickel slag cementitious materials. Constr. Build. Mater. 2020, 235, 117449. [Google Scholar] [CrossRef]
- Boudchicha, M.R.; Rubio, F.; Achour, S. Synthesis of glass ceramics from kaolin and dolomite mixture. Int. J. Miner. Metall. Mater. 2017, 24, 194–201. [Google Scholar] [CrossRef]
- Molla, A.R.; Basu, B. Microstructure, mechanical, and in vitro properties of mica glass-ceramics with varying fluorine content. J. Mater. Sci. Mater. Med. 2009, 20, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.W.; Zhong, J.W.; Mao, Y.R. Effect of CuO on crystallisation and properties of red R2O–CaO–MgO–Al2O3–SiO2 glass-ceramics from granite wastes. Ceram. Int. 2020, 46, 23186–23193. [Google Scholar] [CrossRef]
- Shi, J.; He, F.; Ye, C.Q. Preparation and characterization of CaO–Al2O3–SiO2 glass-ceramics from molybdenum tailings. Mater. Chem. Phys. 2017, 197, 57–64. [Google Scholar] [CrossRef]
- Liu, H.P.; Huang, X.F.; Ma, L.P. Effect of Fe2O3 on the crystallization behavior of glass-ceramics produced from naturally cooled yellow phosphorus furnace slag. Int. J. Miner. Metall. Mater. 2017, 24, 316–323. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, C.X.; Zhou, J.C. Continuous cooling crystallization kinetics of a molten blast furnace slag. J. Non-Cryst. Solids 2012, 358, 20–24. [Google Scholar]
- Zhao, Y.; Chen, D.F.; Bi, Y.Y. Preparation of low cost glass–ceramics from molten blast furnace slag. Ceram. Int. 2012, 38, 2495–2500. [Google Scholar] [CrossRef]
- Jian, P.; Zheng, G.L.; Zhu, D.Q. Utilization of nickel slag using selective reduction followed by magnetic separation. Trans. Nonferr. Met. Soc. China 2013, 23, 3421–3427. [Google Scholar]
- Li, X.M.; Zhang, X.Y.; Li, Y. Isothermal oxidation kinetics and oxidation behavior of nickel slag. Metall. Res. Technol. 2020, 117, 603. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, L.J.; Ma, M.S. Effect of Nucleating Agents on Crystallization Glass Ceramics Produced from Nickel Slag. Adv. Mat. Res. 2010, 915, 773–777. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, X.Y.; Zang, X.Y. Structure and Phase Changes of Nickel Slag in Oxidation Treatment. Minerals 2020, 10, 313. [Google Scholar] [CrossRef] [Green Version]
- Rezvani, M.; Eftekhari, Y.B.; Solati, H.M. Effect of Cr2O3, Fe2O3 and TiO2 nucleants on the crystallization behaviour of SiO2–Al2O3–CaO–MgO(R2O) glass-ceramics. Ceram. Int. 2004, 31, 75–80. [Google Scholar] [CrossRef]
- Zhao, M.Z.; Cao, J.W.; Wang, Z. Insight into the dual effect of Fe2O3 addition on the crystallization of CaO–MgO–Al2O3–SiO2 glass-ceramics. J. Non-Cryst. Solids 2019, 513, 144–151. [Google Scholar] [CrossRef]
- Pei, F.J.; Zhu, G.H.; Li, P. Effects of CaF2 on the sintering and crystallisation of CaO–MgO–Al2O3–SiO2 glass-ceramics. Ceram. Int. 2020, 46, 17825–17835. [Google Scholar] [CrossRef]
- Reben, M.; Kosmal, M.; Ziąbka, P. The influence of TiO2 and ZrO2 on microstructure and crystallization behavior of CRT glass. J. Non-Cryst. Solids 2015, 425, 118–123. [Google Scholar] [CrossRef]
- Maeyer, E.D.; Verbeeck, R.; Vercruysse, C. Infrared spectrometric study of acid-degradable glasses. J. Dent. Res. 2002, 81, 552–555. [Google Scholar] [CrossRef]
- Yang, Z.H.; Lin, Q.; Lu, S.C. Effect of CaO/SiO2 ratio on the preparation and crystallization of glass-ceramics from copper slag. Ceram. Int. 2014, 40, 7297–7305. [Google Scholar] [CrossRef]
- Tomohiro, T.; Aiko, N.; Yoshikazu, K. Glass-ceramics prepared from sludge generated by a water purification plant. Ceram. Int. 2007, 33, 573–577. [Google Scholar]
- Wang, W.W.; Liu, C.; Shi, L. Effects of Li2O–B2O3–SiO2–CaO–Al2O3 glass addition on the sintering behavior and microwave dielectric properties of Li3Mg2NbO6 ceramics. Appl. Phys. 2019, 125, 602. [Google Scholar] [CrossRef]
- Cannas, C.; Musinu, A.; Piccaluga, G. Advances in the structure and microstructure determination of yttrium silicates using the Rietveld method. J. Solid State Chem. 2005, 178, 1526–1532. [Google Scholar] [CrossRef]
- Zou, C.M.; Cao, J.W.; Zhao, M.Z. Combined sodium and fluorine promote diopside continuous growth to achieve one-step crystallization in CaO–Al2O3–SiO2–Fe2O3 glass-ceramics. J. Eur. Ceram. Soc. 2019, 39, 4979–4987. [Google Scholar] [CrossRef]
- Zhao, S.Z.; Liu, B.; Ding, Y.J. Study on glass-ceramics made from MSWI fly ash, pickling sludge and waste glass by one-step process. J. Clean. Prod. 2020, 271, 122674. [Google Scholar] [CrossRef]
- Alizadeh, P.; Yekta, B.E. Effect of Fe2O3 addition on the sinterability and machinability of glass-ceramics in the system MgO–CaO–SiO2–P2O5. J. Eur. Ceram. Soc. 2004, 24, 3529–3533. [Google Scholar] [CrossRef]
- Noelio, O.; Walter, E.; Anielle, C.A. Effect of Fe2O3 concentration on the structure of the SiO2–Na2O–Al2O3–B2O3 glass system. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 81, 140–143. [Google Scholar]
- Nagarjuna, G.; Enkatramaiah, N.; Satyanarayana, P.V.V. Fe2O3-induced crystallization and the physical properties of lead arsenate glass system. J. Alloys Compd. 2009, 468, 466–476. [Google Scholar] [CrossRef]
- Jeff, K.; Merete, T.; Gabriella, T. A Raman Spectroscopic Study of the Structural Modifications Associated with the Addition of Calcium Oxide and Boron Oxide to Silica. Metall. Mater. Trans. B 2015, 46, 62–73. [Google Scholar]
- Molla, A.R.; Kesavulu, C.R.; Chakradhar, R. Microstructure, mechanical, thermal, EPR, and optical properties of MgAl2O4:Cr3+ spinel glass–ceramic nanocomposites. J. Alloys Compd. 2014, 583, 498–509. [Google Scholar] [CrossRef]
- Xing, X.D.; Du, Y.L.; Zheng, J.L. Isothermal Carbothermal Reduction of FeTiO3 Doped with MgO. JOM 2021, 73, 1328–1336. [Google Scholar] [CrossRef]
- Kang, J.F.; Chen, Z.Y.; Zhu, X.G. Effect of replacement of Na2O by Fe2O3 on the crystallization behavior and acid resistance of MgO–Al2O3–SiO2 glass-ceramics. J. Non-Cryst. Solids 2018, 503–504, 1–6. [Google Scholar]
- Anusavice, K.J.; Zhang, N.Z. Effect of Crystallinity on Strength and Fracture Toughness of Li2O–Al2O3–CaO–SiO2 Glass-Ceramics. J. Am. Ceram. Soc. 2010, 80, 1353–1358. [Google Scholar] [CrossRef]










| TFe | CaO | SiO2 | MgO | Al2O3 | Na2O | K2O | Other | |
|---|---|---|---|---|---|---|---|---|
| Nickel slag | 36.24 | 3.35 | 28.32 | 9.86 | 2.52 | 0.56 | 0.25 | 3.37 |
| Blast furnace slag | - | 41.13 | 39.17 | 8.00 | 9.50 | - | - | 2.20 |
| CaO | SiO2 | MgO | Al2O3 | Fe2O3 | The Reduction Rate (%) |
|---|---|---|---|---|---|
| 21.39 | 54.59 | 16.70 | 7.32 | 0 | 100 |
| 21.39 | 54.59 | 16.70 | 7.32 | 3.11 | 90 |
| 21.39 | 54.59 | 16.70 | 7.32 | 6.21 | 80 |
| 21.39 | 54.59 | 16.70 | 7.32 | 9.32 | 70 |
| 21.39 | 54.59 | 16.70 | 7.32 | 12.42 | 60 |
| Structural Units | Onb | Qn | IR Band (cm−1) |
|---|---|---|---|
| [SiO4]4− | 4 | Q0 | 840–890 |
| [Si2O7]6− | 3 | Q1 | 900–950 |
| [SiO3]2− | 2 | Q2 | 960–1130 |
| [Si2O5]2− | 1 | Q3 | 1050–1100 |
| [SiO2]0 | 0 | Q4 | 1160–1190 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zang, X.; Xing, X.; Li, J.; Ma, Y.; Li, T. Effect of Fe2O3 on the Crystallization Behavior of Glass-Ceramics Produced from Secondary Nickel Slag. Metals 2022, 12, 164. https://doi.org/10.3390/met12010164
Li X, Zang X, Xing X, Li J, Ma Y, Li T. Effect of Fe2O3 on the Crystallization Behavior of Glass-Ceramics Produced from Secondary Nickel Slag. Metals. 2022; 12(1):164. https://doi.org/10.3390/met12010164
Chicago/Turabian StyleLi, Xiaoming, Xuyuan Zang, Xiangdong Xing, Jinke Li, Yuwei Ma, and Tao Li. 2022. "Effect of Fe2O3 on the Crystallization Behavior of Glass-Ceramics Produced from Secondary Nickel Slag" Metals 12, no. 1: 164. https://doi.org/10.3390/met12010164