The Emergence of the Family Scirtidae (Insecta: Coleoptera) in Lotic Karst Habitats: A Case Study over 15 Years
Abstract
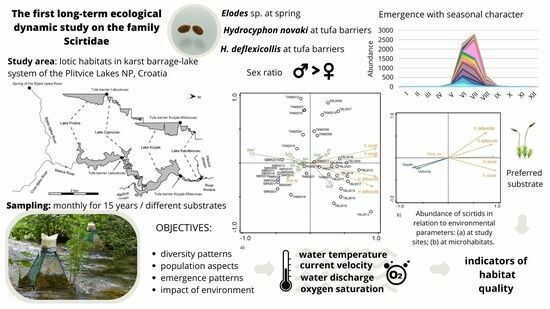
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Sampling and Identification
2.3. Abiotic and Biotic Environmental Parameters
2.4. Data Analysis
3. Results
3.1. Faunal Assemblage and Distribution
3.2. Sex Ratio and Emergence Patterns
3.3. Environmental Characteristics and Scirtids
4. Discussion
4.1. Faunal Asemblage and Distribution
4.2. Sex Ratio and Emergence Patterns
4.3. Environmental Characteristics and Scirtids
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jäch, M.A. Annotated Check List of Aquatic and Riparian/Littoral Beetle Families of the World. In Water Beetles of China; Jäch, M.A., Ji, L., Eds.; Zoologisch-Botanische Gesellschaft in Österreich und Wiener Koleopterologen Verein: Wien, Austria, 1998; pp. 25–42. [Google Scholar]
- Jäch, M.A.; Balke, M. Global Diversity of Water Beetles (Coleoptera) in Freshwater. Hydrobiologia 2008, 595, 419–442. [Google Scholar] [CrossRef]
- Short, A.E.Z. Systematics of Aquatic Beetles (Coleoptera): Current State and Future Directions. Syst. Entomol. 2018, 43, 1–18. [Google Scholar] [CrossRef]
- Brojer, M.; Jäch, M.; Kodada, J.; Moog, O. Coleoptera: Water Beetles Sl. In Fauna Aquatica Austriaca. A Comprehensive Species Inventory of Austrian Aquatic Organisms with Ecological Notes; Moog, O., Hartmann, A., Eds.; Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft: Wien, Austria, 2017; p. 49. [Google Scholar]
- Sánchez-Fernández, D.; Abellán, P.; Velasco, J.; Millán, A. Selecting Areas to Protect the Biodiversity of Aquatic Ecosystems in a Semiarid Mediterranean Region Using Water Beetles. Aquat. Conserv. Mar. Freshw. 2004, 14, 465–479. [Google Scholar] [CrossRef]
- Abellán, P.; Sánchez-Fernández, D.; Velasco, J.; Millán, A. Assessing Conservation Priorities for Insects: Status of Water Beetles in Southeast Spain. Biol. Conserv. 2005, 121, 79–90. [Google Scholar] [CrossRef]
- Elliott, J.M. The Ecology of Riffle Beetles (Coleoptera: Elmidae). Freshw. Rev. 2008, 1, 189–203. [Google Scholar] [CrossRef]
- Libonatti, M.L.; Ruta, R. Family Scirtidae. In Thorp and Covich’s Freshwater Invertebrates; Elsevier: Amsterdam, The Netherlands, 2018; pp. 599–603. ISBN 978-0-12-804223-6. [Google Scholar]
- Ruta, R.; Klausnitzer, B.; Prokin, A. South American Terrestrial Larva of Scirtidae (Coleoptera: Scirtoidea): The Adaptation of Scirtidae Larvae to Saproxylic Habitat Is More Common than Expected: Terrestrial Scirtidae Larvae. Austral Entomol. 2017, 57, 50–61. [Google Scholar] [CrossRef]
- Klečka, J. The Structure and Dynamics of a Water Beetle Community in a Semipermanent Wetland (Vrbenské Rybníky Nature Reserve, South Bohemia). Master’s Thesis, The University of South Bohemia, České Budějovice, Czech Republic, 2008. [Google Scholar]
- Mičetić Stanković, V.; Jäch, M.A.; Ivković, M.; Stanković, I.; Kružić, P.; Kučinić, M. Spatio-Temporal Distribution and Species Traits of Water Beetles along an Oligotrophic Hydrosystem: A Case Study. Ann. Limnol.-Int. J. Lim. 2019, 55, 22. [Google Scholar] [CrossRef]
- Nadein, K.; Kovalev, A.; Gorb, S. Jumping Mechanism in the Marsh Beetles (Coleoptera: Scirtidae). Sci. Rep. 2022, 12, 15834. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, T. Zur Morphologie und Funktion des Helodiden-Aedoeagus (Col.). Insect Syst. Evol. 1972, 3, 81–119. [Google Scholar] [CrossRef]
- Jolivet, P. Inverted Copulation. In Encyclopedia of Entomology; Capinera, J.L., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 2014–2044. [Google Scholar]
- Zwick, P.; Klausnitzer, B.; Ruta, R. Contacyphon Gozis, 1886 Removed from Synonymy (Coleoptera: Scirtidae) to Accommodate Species so Far Combined with the Invalid Name, Cyphon Paykull, 1799. Entomol. Bl. Coleopt. 2013, 109, 337–353. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity Hotspots for Conservation Priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Ivković, M.; Plant, A. Aquatic Insects in the Dinarides: Identifying Hotspots of Endemism and Species Richness Shaped by Geological and Hydrological History Using Empididae (Diptera). Insect Conserv. Divers. 2015, 8, 302–312. [Google Scholar] [CrossRef]
- Bonacci, O. Karst Hydrology and Water Resources—Past, Present and Future. In Water for the Future: Hydrology in Perspective; Rodda, J.C., Matalas, N.C., Eds.; International Association of Hydrological Sciences: Rome, Italy, 1987; pp. 205–213. [Google Scholar]
- Bonacci, O.; Pipan, T.; Culver, D.C. A Framework for Karst Ecohydrology. Environ. Geol. 2009, 56, 891–900. [Google Scholar] [CrossRef]
- Giłka, W.; Zakrzewska, M.; Baranov, V.A.; Dominiak, P. Diagnostic Clues for Identification of Selected Species of the Micropsectra Atrofasciata Group, with Description of M. Uva Sp. Nov. from Croatia (Diptera: Chironomidae: Tanytarsini). Zootaxa 2013, 3702, 288. [Google Scholar] [CrossRef] [PubMed]
- Kvifte, G.M.; Ivković, M. New Species and Records of the Pericoma Trifasciata Group from Croatia (Diptera: Psychodidae). Zootaxa 2018, 4486, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Baranov, V.; Hagenlund, L.K.; Ivković, M.; Kvifte, G.M.; Pavlek, M. Blind Flight? A New Troglobiotic Orthoclad (Diptera, Chironomidae) from the Lukina Jama–Trojama Cave in Croatia. PLoS ONE 2016, 11, e0152884. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.M.; Kryštufek, B.; Eastwood, W.J. The Physical Geography of the Balkans and Nomenclature of Place Names. In Balkan Biodiversity: Pattern and Process in the European Hotspot; Springer: Dordrecht, The Netherlands, 2004; pp. 9–22. [Google Scholar]
- Miliša, M.; Ivković, M. Plitvice Lakes; Springer Water; Springer International Publishing: Cham, Switzerland, 2023; ISBN 978-3-031-20377-0. [Google Scholar]
- Schlosser, J.K. Fauna Kornjaśah Trojedne Kraljevine; Jugoslavenska Akademija Znanosti i Umjetnosti: Zagreb, Croatia, 1878; Volume 2. [Google Scholar]
- Koča, G. Popis Tvrdokrilaca (Kornjaša) Vinkovačke Okolice. Glas. Hrvat. Naravosl. Druš. 1905, 17, 119–212. [Google Scholar]
- Matoničkin, I.; Pavletić, Z. Hidrologija Protočnog Sistema Plitvičkih Jezera i Njegove Ekološko-Biocenološke Značajke. Carsus Iugosl. 1967, 5, 83–126. [Google Scholar]
- Habdija, I.; Primc-Habdija, B.; Belinić, I. Functional Community Organization of Macroinvertebrates in Lotic Habitats of the Plitvice Lakes. Acta Hydrochim. Hydrobiol. 1994, 22, 85–92. [Google Scholar] [CrossRef]
- Novak, P. Kornjaši Jadranskog Primorja; Jugoslavenska Akademija Znanosti i Umjetnosti: Zagreb, Croatia, 1952. [Google Scholar]
- Klausnitzer, B. Scirtidae. In Catalogue of Palaearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Denmark, 2006. [Google Scholar]
- Mičetić Stanković, V.; Jäch, M.A.; Vučković, I.; Popijač, A.; Kerovec, M.; Kučinić, M. Ecological Traits of Water Beetles in a Karstic River from the Eastern Mediterranean Region. Limnologica 2018, 71, 75–88. [Google Scholar] [CrossRef]
- Ivković, M.; Mičetić Stanković, V.; Mihaljević, Z. Emergence Patterns and Microhabitat Preference of Aquatic Dance Flies (Empididae; Clinocerinae and Hemerodromiinae) on a Longitudinal Gradient of Barrage Lake System. Limnologica 2012, 42, 43–49. [Google Scholar] [CrossRef]
- Ivković, M.; Kesić, M.; Mihaljević, Z.; Kúdela, M. Emergence Patterns and Ecological Associations of Some Haematophagous Blackfly Species along an Oligotrophic Hydrosystem: Emergence and Ecology of Blackflies. Med. Vet. Entomol. 2014, 28, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kučinić, M.; Previšić, A.; Vajdić, M.; Tunjić, M.; Mihoci, I.; Žalac, S.; Sviben, S.; Vućković, I.; Trupković, M.; Habdija, I. First systematic investigation of adults and second checklist of caddisflies of the Plitvice Lakes National Park with notes on research history, biodiversity, distribution and ecology. Nat. Croat. 2017, 26, 225–260. [Google Scholar] [CrossRef]
- Vilenica, M.; Ivković, M. A Decade-Long Study on Mayfly Emergence Patterns. Mar. Freshw. Res. 2021, 72, 507. [Google Scholar] [CrossRef]
- Ridl, A.; Vilenica, M.; Ivković, M.; Popijač, A.; Sivec, I.; Miliša, M.; Mihaljević, Z. Environmental Drivers Influencing Stonefly Assemblages along a Longitudinal Gradient in Karst Lotic Habitats. J. Limnol. 2018, 77, 412–427. [Google Scholar] [CrossRef]
- Baranov, V.; Jourdan, J.; Pilotto, F.; Wagner, R.; Haase, P. Complex and Nonlinear Climate-driven Changes in Freshwater Insect Communities over 42 Years. Conserv. Biol. 2020, 34, 1241–1251. [Google Scholar] [CrossRef] [PubMed]
- Dorić, V.; Ivković, M.; Baranov, V.; Pozojević, I.; Mihaljević, Z. Extreme Freshwater Discharge Events Exacerbated by Climate Change Influence the Structure and Functional Response of the Chironomid Community in a Biodiversity Hotspot. Sci. Total Environ. 2023, 879, 163110. [Google Scholar] [CrossRef]
- Pozojević, I.; Dorić, V.; Miliša, M.; Ternjej, I.; Ivković, M. Defining Patterns and Rates of Natural vs. Drought Driven Aquatic Community Variability Indicates the Ongoing Need for Long Term Ecological Research. Biology 2023, 12, 590. [Google Scholar] [CrossRef]
- Ivanković, L.; Ivković, M.; Stanković, I. Perennial Phenology Patterns and Ecological Traits of Dixidae (Insecta, Diptera) in Lotic Habitats of a Barrage Lake System. Limnologica 2019, 76, 11–18. [Google Scholar] [CrossRef]
- Makjanić, B. O Klimi Užeg Područja Plitvičkih Jezera. Hrvat. Geogr. Glas. 1971, 33, 5–23. [Google Scholar]
- Šegota, T.; Filipčić, A. Köppenova Podjela Klima i Hrvatsko Nazivlje. Geoadria 2003, 8, 17–37. [Google Scholar] [CrossRef]
- Poje, D. Pregled Klimatskih Karakteristika Nacionalnog Parka Plitvička Jezera. Plitvički Bilt. 1989, 2, 87–99. [Google Scholar]
- Bočić, N.; Barudžija, U.; Pahernik, M. Geomorphological and Geological Properties of Plitvice Lakes Area. In Plitvice Lakes; Miliša, M., Ivković, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 1–16. [Google Scholar]
- Matoničkin Kepčija, R.; Miliša, M. Recent Tufa Deposition. In Plitvice Lakes; Miliša, M., Ivković, M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 123–144. [Google Scholar]
- Tabashnik, B.E. Population Structure of Pierid Butterflies: III. Pest Populations of Colias Philodice Eriphyle. Oecologia 1980, 47, 175–183. [Google Scholar] [CrossRef]
- Hurlbert, S.H. Pseudoreplication and the Design of Ecological Field Experiments. Ecol. Monogr. 1984, 54, 187–211. [Google Scholar] [CrossRef]
- Clarke, K.R. Detecting Change in Benthic Community Structure. In Proceedings of the Invited Papers, 14th International Biometric Conference; Oger, R., Ed.; Société Adolphe Quetelet: Namour, Belgium, 1988; pp. 131–142. [Google Scholar]
- Clarke, K.R. Non-parametric Multivariate Analyses of Changes in Community Structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- SPSS Inc. SPSS Statistics, 17.0. Windows; SPSS Inc.: Chicago, IL, USA, 2008. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User’s Guide: Software for Ordination, Version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Ter Braak, C.; Šmilauer, P. CANOCO 5, version 5.0. Windows; Biometrics Plant Research International: Wageningen, The Netherlands, 2012. [Google Scholar]
- Adobe Systems Incorporated. Adobe® Illustrator®, CS6. Windows; Adobe Systems Incorporated: San Jose, CA, USA, 2012. [Google Scholar]
- Golden Software, LLC. GrapherTM20. Windows; Golden Software, LLC.: Golden, CO, USA, 2022. [Google Scholar]
- Cooper, S.J.; Watts, C.H.; Saint, K.M.; Leijs, R. Phylogenetic Relationships of Australian Scirtidae (Coleoptera) Based on Mitochondrial and Nuclear Sequences. Invertebr. Syst. 2014, 28, 628–642. [Google Scholar] [CrossRef]
- Watts, C.; Bradford, T.; Cooper, S. A New Genus, Perplexacara, and New Generic Placements of Species of Australian Marsh Beetles (Coleoptera: Scirtidae) Based on Morphology and Molecular Genetic Data. Zootaxa 2021, 4927, zootaxa.4927.4.4. [Google Scholar] [CrossRef]
- Cuppen, J. Flight Periods of Scirtidae (Coleoptera) Based on Weekly Samples from a Malaise Trap. Entomol. Ber. 1993, 53, 137–142. [Google Scholar]
- Paradise, C.J.; Kuhn, K.L. Interactive Effects of pH and Leaf Litter on a Shredder, the Scirtid Beetle, Helodes Pulchella, Inhabiting Tree-holes. Freshw. Biol. 1999, 41, 43–49. [Google Scholar] [CrossRef]
- Daugherty, M.P.; Juliano, S.A. Factors Affecting the Abundance of Scirtid Beetles in Container Habitats. J. N. Am. Benthol. Soc. 2001, 20, 109–117. [Google Scholar] [CrossRef]
- Matoničkin, I.; Pavletić, Z.; Tavčar, V.; Krkač, N. Limnološka Istraživanja Reikotopa i Fenomena Protočne Travertinizacije u Plitvičkim Jezerima. Acta Biol. 1971, 7, 1–88. [Google Scholar]
- Mičetić Stanković, V. Vodeni Kornjaši (Insecta:Coleoptera) u Mikrostaništima Krških Izvora i Tekućica. Doktorska Disertacija, Sveučilište u Zagrebu, Prirodoslovno, Matematički Fakultet, Zagreb, Croatia, 2012. [Google Scholar]
- Miliša, M.; Habdija, I.; Primc-Habdija, B.; Radanović, I.; Kepčija, R.M. The Role of Flow Velocity in the Vertical Distribution of Particulate Organic Matter on Moss-Covered Travertine Barriers of the Plitvice Lakes (Croatia). Hydrobiologia 2006, 553, 231–243. [Google Scholar] [CrossRef]
- Sertić Perić, M.; Matoničkin Kepčija, R.; Radanović, I.; Primc, B.; Habdija, I. Freshwater Reefs as Mesohabitats for the Assessment of Diel Invertebrate Drift Patterns. Nat. Croat. 2020, 29, 185–203. [Google Scholar] [CrossRef]
- Habdija, I.; Primc Habdija, B.; Matonickin, R.; Kucinic, M.; Radanovic, I.; Milisa, M.; Mihaljevic, Z. Current Velocity and Food Supply as Factors Affecting the Composition of Macroinvertebrates in Bryophyte Habitats in Karst Running Water. Biologia 2004, 59, 577–594. [Google Scholar]
- Plenkovic-Moraj, A.; Horvatincic, N.; Primc-Habdija, B. Periphyton and Its Role in Tufa Deposition in Karstic Waters (Plitvice Lakes, Croatia). Biologia 2002, 57, 423–431. [Google Scholar]
- Klausnitzer, B. Insecta: Coleoptera: Scirtidae; Spektrum Akademischer Verlag: Heidelberg, Germany, 2009; ISBN 978-3-8274-1074-0. [Google Scholar]
- Moog, O.; Hartmann, A. Fauna Aquatica Austriaca, 3rd Edition 2017. A Comprehensive Species Inventory of Austrian Aquatic Organisms with Ecological Notes; Wasserwirtschaftskataster, Bundesministerium für Land-und Forstwirtschaft: Wien, Austria, 2017; ISBN 978-3-85174-074-5. [Google Scholar]
- Minshall, G.W. Aquatic Insect-Substratum Relationships. In The Ecology of Aquatic Insects; Resh, V.H., Rosenberg, D.M., Eds.; Praeger Scientific: New York, NY, USA, 1984; pp. 358–400. [Google Scholar]
- Mičetić Stanković, V.; Bruvo Mađarić, B.; Kučinić, M. Ubiquitous but Ignored? A Case of Water Beetle in Southeastern Europe. Diversity 2022, 14, 26. [Google Scholar] [CrossRef]
- Juliano, S.A. Quantitative Analysis of Sexual Dimorphism and Sex Ratio in Hyphydrus Ovatus (Coleoptera: Dytiscidae). Ecography 1992, 15, 308–313. [Google Scholar] [CrossRef]
- Corbet, P.S. Temporal Patterns of Emergence in Aquatic Insects. Can. Entomol. 1964, 96, 264–279. [Google Scholar] [CrossRef]
- Ivković, M.; Pont, A.C. Long-Time Emergence Patterns of Limnophora Species (Diptera, Muscidae) in Specific Karst Habitats: Tufa Barriers. Limnologica 2016, 61, 29–35. [Google Scholar] [CrossRef]
- Vilenica, M. Ecological Traits of Dragonfly (Odonata) Assemblages along an Oligotrophic Dinaric Karst Hydrosystem. Ann. Limnol.-Int. J. Lim. 2017, 53, 377–389. [Google Scholar] [CrossRef]
- Pozojević, I.; Ivković, M.; Cetinić, K.A.; Previšić, A. Peeling the Layers of Caddisfly Diversity on a Longitudinal Gradient in Karst Freshwater Habitats Reveals Community Dynamics and Stability. Insects 2021, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Hogg, I.D.; Williams, D.D. Response of Stream Invertebrates to a Global-warming Thermal Regime: An Ecosystem-level Manipulation. Ecology 1996, 77, 395–407. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Vannote, R.L. Ephemerella Mayflies of White Clay Creek: Bioenergetic and Ecological Relationships among Six Coexisting Species. Ecology 1981, 62, 1353–1369. [Google Scholar] [CrossRef]
- Sweeney, B.W. Factors Influencing Life-History Patterns of Aquatic Insects. In The Ecology of Aquatic Insects; Resh, V.H., Rosenberg, D.M., Eds.; Praeger Scientific: New York, NY, USA, 1984; pp. 56–100. [Google Scholar]
- Bournaud, M.; Richoux, P.; Usseglio-Polatera, P. An Approach to the Synthesis of Qualitative Ecological Information from Aquatic Coleoptera Communities. Regul. Rivers Res. Manag. 1992, 7, 165–180. [Google Scholar] [CrossRef]






| Site | SBR | TBL | TKM |
|---|---|---|---|
| Latitude | N 44°50′05″ | N 44°52′17″ | N 44°53′39″ |
| Longitude | E 15°33′43″ | E 15°35′59″ | E 15°36′32″ |
| Altitude (m) | 720 | 630 | 546 |
| Water temperature (°C) | |||
| min | 7.2 | 1.663 | 1.67 |
| max | 8.4 | 20.63 | 22.95 |
| O2 (%) | |||
| min | 65.21 | 59.7 | 72.04 |
| max | 106.3 | 139.2 | 130.5 |
| pH | |||
| min | 6.8 | 7.95 | 7.98 |
| max | 8.9 | 8.63 | 8.52 |
| Alkalinity (mg CaCO3 L−1) | |||
| min | 242.2 | 180.94 | 159.5 |
| max | 302.3 | 270.5 | 226.5 |
| Conductivity (µS cm−1) | |||
| min | 384 | 348 | 323 |
| max | 528 | 445 | 415 |
| NO3 (mg N L−1) | |||
| min | 0 | 0.01 | 0.01 |
| max | 1.58 | 1.06 | 1.14 |
| NO2 (mg N L−1) | |||
| min | 0 | 0 | 0 |
| max | 0.002 | 0.008 | 0.01 |
| NH4+ (mg L−1) | |||
| min | 0 | 0 | 0 |
| max | 0.2 | 0.22 | 0.103 |
| PO4− (mg L−1) | |||
| min | 0 | 0 | 0 |
| max | 0.049 | 0.07 | 0.255 |
| Water discharge (m3 s−1) | |||
| min | 0.15 | 1.74 | 1.4 |
| max | 1.07 | 5.58 | 4.22 |
| Emergence trap/substrate type | |||
| P1 | gravel and sand | pebbles | pebbles |
| P2 | gravel and sand | pebbles | fine silt and sand |
| P3 | bryophytes | bryophytes | pebbles |
| P4 | angiosperms | pebbles | bryophytes |
| P5 | bryophytes | bryophytes | bryophytes |
| P6 | angiosperms | bryophytes | fine silt and sand |
| P7 | - | bryophytes | - |
| Current velocity (m3 s−1) | |||
| min | 0 | 1.2 | 0 |
| max | 37 | 50.56 | 48.66 |
| Depth (m) | |||
| min | 0.06 | 0.08 | 0.08 |
| max | 0.25 | 0.45 | 0.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klarin, A.; Ivković, M.; Mičetić Stanković, V. The Emergence of the Family Scirtidae (Insecta: Coleoptera) in Lotic Karst Habitats: A Case Study over 15 Years. Insects 2024, 15, 226. https://doi.org/10.3390/insects15040226
Klarin A, Ivković M, Mičetić Stanković V. The Emergence of the Family Scirtidae (Insecta: Coleoptera) in Lotic Karst Habitats: A Case Study over 15 Years. Insects. 2024; 15(4):226. https://doi.org/10.3390/insects15040226
Chicago/Turabian StyleKlarin, Ana, Marija Ivković, and Vlatka Mičetić Stanković. 2024. "The Emergence of the Family Scirtidae (Insecta: Coleoptera) in Lotic Karst Habitats: A Case Study over 15 Years" Insects 15, no. 4: 226. https://doi.org/10.3390/insects15040226