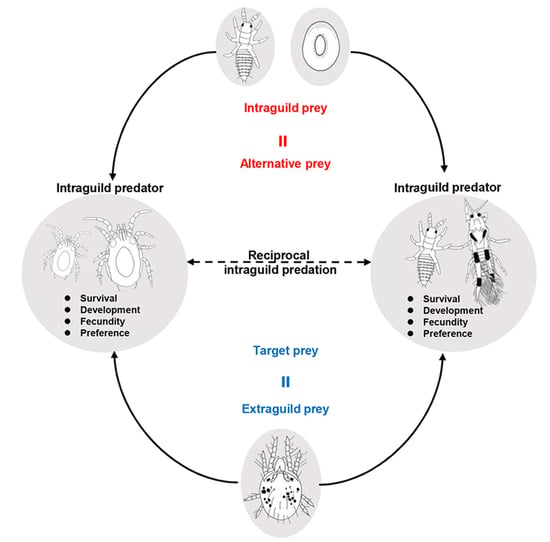
Intraguild Prey Served as Alternative Prey for Intraguild Predators in a Reciprocal Predator Guild between Neoseiulus barkeri and Scolothrips takahashii
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Rearing of Mites and Thrips
2.2. Experimental Design and Procedures
2.2.1. Survival and Development of Immature Intraguild Predators
2.2.2. Survival and Oviposition of Adult Female Intraguild Predators
2.2.3. Prey Preference of Intraguild Predators
2.3. Data Analysis
3. Results
3.1. Survival and Development of Immature Intraguild Predators
3.2. Survival and Oviposition of Adult Female Intraguild Predators
3.3. Prey Preference of Intraguild Predators
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Messelink, G.J.; Janssen, A. Increased control of thrips and aphids in greenhouses with two species of generalist predatory bugs involved in intraguild predation. Biol. Control 2014, 79, 1–7. [Google Scholar] [CrossRef]
- Sabelis, M.W.; Hanna, R.; Onzo, A.; Pallini, A.; Cakmak, I.; Janssen, A. Multiple predators, intraguild interactions and biological control of a single spider mite species. Integr. Control Plant-Feed. Mites IOBC/Wprs Bull. 2009, 50, 83–94. [Google Scholar]
- Polis, G.A.; Myers, C.A.; Holt, R.D. The ecology and evolution of intraguild predation: Potential competitors that eat each other. Annu. Rev. Ecol. Syst. 1989, 20, 297–330. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Kaya, H.K.; Ehler, L.E.; Marois, J.J.; Jaffee, B.A. Intraguild predation among biological-control agents: Theory and evidence. Biol. Control 1995, 5, 303–335. [Google Scholar] [CrossRef]
- Polis, G.A. The evolution and dynamics of intraspecific predation. Annu. Rev. Ecol. Syst. 1981, 12, 225–251. [Google Scholar] [CrossRef]
- Denno, R.F.; Finke, D.L. Multiple predator interactions and food-web connectance: Implications for biological control. In Trophic and Guild in Biological Interactions Control; Brodeur, J., Boivin, G., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 45–70. [Google Scholar]
- Mirande, L.; Desneux, N.; Haramboure, M.; Schneider, M.I. Intraguild predation between an exotic and a native coccinellid in Argentina: The role of prey density. J. Pest Sci. 2015, 88, 155–162. [Google Scholar] [CrossRef]
- Bahlai, C.A.; Colunga-Garcia, M.; Gage, S.H.; Landis, D.A. The role of exotic ladybeetles in the decline of native ladybeetle populations: Evidence from long-term monitoring. Biol. Invasions 2015, 17, 1005–1024. [Google Scholar] [CrossRef]
- Döker, İ.; Revynthi, A.M.; Kazak, C.; Carrillo, D. Interactions among exotic and native phytoseiids (Acari: Phytoseiidae) affect biocontrol of two-spotted spider mite on papaya. Biol. Control 2021, 163, 104758. [Google Scholar] [CrossRef]
- Montserrat, M.; Magalhães, S.; Sabelis, M.W.; De Roos, A.M.; Janssen, A. Patterns of exclusion in an intraguild predator–prey system depend on initial conditions. J. Anim. Ecol. 2008, 77, 624–630. [Google Scholar] [CrossRef]
- Marques, R.V.; Sarmento, R.A.; Oliveira, A.G.; Rodrigues, D.D.M.; Venzon, M.; PedroNeto, M.; Pallini, A.; Janssen, A. Reciprocal intraguild predation and predator coexistence. Ecol. Evol. 2018, 8, 6952–6964. [Google Scholar] [CrossRef]
- Bilu, E.; Coll, M. The importance of intraguild interactions to the combined effect of a parasitoid and a predator on aphid population suppression. BioControl 2007, 52, 753–763. [Google Scholar] [CrossRef]
- Mirhosseini, M.A.; Fathipour, Y.; Holst, N.; Soufbaf, M.; Michaud, J.P. An egg parasitoid interferes with biological control of tomato leafminer by augmentation of Nesidiocoris tenuis (Hemiptera: Miridae). Biol. Control 2019, 133, 34–40. [Google Scholar] [CrossRef]
- Michalko, R.; Uhrinec, M.; Khum, W.; Sentenská, L. The benefits of intraguild predation for a top predator spider. Ecol. Entomol. 2021, 46, 283–291. [Google Scholar] [CrossRef]
- Fonseca, M.M.; Montserrat, M.; Guzmán, C.; Torres-Campos, I.; Pallini, A.; Janssen, A. How to evaluate the potential occurrence of intraguild predation. Exp. Appl. Acarol. 2017, 72, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onzo, A.; Hanna, R.; Negloh, K.; Toko, M.; Sabelis, M.W. Biological control of cassava green mite with exotic and indigenous phytoseiid predators—Effects of intraguild predation and supplementary food. Biol. Control 2005, 33, 143–152. [Google Scholar] [CrossRef]
- Walzer, A.; Schausberger, P. Predation preferences and discrimination between con- and heterospecific prey by the phytoseiid mites Phytoseiulus persimilis and Neoseiulus californicus. BioControl 1999, 43, 469–478. [Google Scholar] [CrossRef]
- Schausberger, P.; Croft, B.A. Nutritional benefits of intraguild predation and cannibalism among generalist and specialist phytoseiid mites. Ecol. Entomol. 2000, 25, 473–480. [Google Scholar] [CrossRef]
- Buitenhuis, R.; Shipp, L.; Scott-Dupree, C. Intra-guild vs extra-guild prey: Effect on predator fitness and preference of Amblyseius swirskii (Athias-Henriot) and Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae). Bull. Entomol. Res. 2010, 100, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Gu, X.Y.; Gui, S.H.; Guo, J.J.; Yi, T.C.; Jin, D.C. Transcriptome analysis of hormone-and cuticle-related genes in the development process of deutonymph in Tetranychus urticae. Insects 2021, 12, 736. [Google Scholar] [CrossRef]
- Li, Y.Y.; Yuan, J.G.; Liu, M.X.; Zhang, Z.H.; Zhou, H.W.; Liu, H. Evaluation of four artificial diets on demography parameters of Neoseiulus barkeri. BioControl 2021, 66, 789–802. [Google Scholar] [CrossRef]
- Yanagita, H.; Morita, S.; Kunimaru, K.; Takemoto, H. Capability of Scolothrips takahashii (Thysanoptera: Thripidae) as a control agent of Tetranychus urticae (Acari: Tetranychidae) for protecting strawberry plug plants in summer. Appl. Entomol. Zool. 2014, 49, 437–441. [Google Scholar] [CrossRef]
- Farazmand, A.; Fathipour, Y.; Kamali, K. Cannibalism in Scolothrips longicornis (Thysanoptera: Thripidae), Neoseiulus californicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae). Syst. Appl. Acarol. 2014, 19, 471–480. [Google Scholar]
- Li, D.X.; Tian, J.; Shen, Z.R. Functional response of the predator Scolothrips takahashii to hawthorn spider mite, Tetranychus viennensis: Effect of age and temperature. BioControl 2007, 52, 41–61. [Google Scholar]
- Li, Y.Y.; Tian, C.B.; Wu, Y.X.; Niu, T.D.; Chu, W.Q.; Liu, H. Molecular characterization of two MAPK genes and their thermotolerant functions in a high temperature acclimated strain of Neoseiulus barkeri. BioControl 2022, 67, 189–200. [Google Scholar]
- Li, Y.Y.; Tian, C.B.; Wu, Y.X.; Niu, T.D.; Wang, H.; Fan, W.H.; Liu, H. Enhanced expression of DNA methyltransferase 1-associated protein1 gene thermotolerance in a high-temperature acclimated predatory mite Neoseiulus barkeri. BioControl 2021, 66, 779–788. [Google Scholar] [CrossRef]
- Liu, M.X.; Chu, W.Q.; Xu, C.; Zheng, Q.M.; Song, W.B.; Li, Y.Y.; Liu, H. Extraguild prey availability reduced cannibalism and reciprocal intraguild predation of Neoseiulus barkeri (Acari: Phytoseiidae) and Scolothrips takahashii (Thysanoptera: Thripidae). Syst. Appl. Acarol. 2020, 25, 775–786. [Google Scholar]
- Zhang, X.X.; Lv, J.L.; Hu, Y.; Wang, B.M.; Chen, X.; Xu, X.N.; Wang, E.D. Prey preference and life table of Amblyseius orientalis on Bemisia tabaci and Tetranychus cinnabarinus. PLoS ONE 2015, 10, 0138820. [Google Scholar] [CrossRef]
- Provost, C.; Lucas, É.; Coderre, D. Prey preference of Hyaliodes vitripennis as an intraguild predator: Active predator choice or passive selection? Biol. Control 2006, 37, 148–154. [Google Scholar] [CrossRef]
- Momen, F.M.; Hussein, H. Influence of prey stage on survival, development and life table of the predacious mite, Neoseiulus barkeri (Hughes) (Acari: Phytoseiidae). Acta Phytopathol. Et Entomol. Hung. 2011, 46, 319–328. [Google Scholar] [CrossRef]
- Li, Y.Y.; Liu, M.X.; Zhou, H.W.; Tian, C.B.; Zhang, G.H.; Liu, Y.Q.; Liu, H.; Wang, J.J. Evaluation of Neoseiulus barkeri (Acari: Phytoseiidae) for control of Eotetranychus kankitus (Acari: Tetranychidae). J. Econ. Entomol. 2017, 110, 903–914. [Google Scholar] [CrossRef]
- Zou, Z.W.; Min, Q.; Xiao, S.G.; Xin, T.R.; Xia, B. Effect of photoperiod on development and demographic parameters of Neoseiulus barkeri (Acari: Phytoseiidae) fed on Tyrophagus putrescentiae (Acari: Acaridae). Exp. Appl. Acarol. 2016, 70, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Zou, Z.W.; Li, P.X.; Lin, P. Effect of temperature on development and reproduction of Neoseiulus barkeri (Acari: Phytoseiidae) fed on Aleuroglyphus ovatus. Exp. Appl. Acarol. 2012, 56, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.H.; Qin, W.J.; Luo, R.H.; Haung, S.J.; Qin, H.G.; Fu, Z.F. Effects of two kinds of pollens on the development and reproduction of Amblyseius barkeri. Plant Prot. 2011, 37, 180–182. [Google Scholar]
- Momen, F.M. Intra- and interspecific predation by Neoseiulus barkeri and Typhlodromus negevi (Acari: Phytoseiidae) on different life stages: Predation rates and effects on reproduction and juvenile development. Acarina 2010, 18, 81–88. [Google Scholar]
- Momen, F.M.; Abdel-Khalek, A. Cannibalism and intraguild predation in the phytoseiid mites Typhlodromips swirskii, Euseius scutalis and Typhlodromus athiasae (Acari: Phytoseiidae). Acarina 2009, 17, 223–229. [Google Scholar]
- Gotoh, T.; Yamaguchi, K.; Fukazawa, M.; Mori, K. Effect of temperature on life history traits of the predatory thrips, Scolothrips takahashii Priesner (Thysanoptera: Thripidae). Appl. Entomol. Zool. 2004, 39, 511–519. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Liu, M.X.; Yuan, J.G.; Okonkwo, T.T.; Chen, H.Q.; Liu, H. Evaluation of a philic egg-consumption predatory thrips Scolothrips takahashii for control of the citrus red mite Panonychus citri. Crop Prot. 2021, 140, 105421. [Google Scholar] [CrossRef]
- Farazmand, A.; Fathipour, Y.; Kamali, K. Intraguild predation among Scolothrips longicornis (Thysanoptera: Thripidae), Neoseiulus californicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae) under laboratory conditions. Insect Sci. 2015, 22, 263–272. [Google Scholar] [CrossRef]
- Weiss, M.R. Defecation behavior and ecology of insects. Annu. Rev. Entomol. 2005, 51, 635–661. [Google Scholar] [CrossRef]
- Bakker, F.M.; Sabelis, M.W. How larvae of Thrips tabaci reduce the attack success of phytoseiid predators. Entomol. Exp. Et Appl. 1989, 50, 47–51. [Google Scholar] [CrossRef]
- De Brujin, P.J.A.; Egas, M.; Janssen, A.; Sabelis, M.W. Pheromone-induced priming of a defensive response in western flower thrips. J. Chem. Ecol. 2006, 32, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Wilder, S.M.; Norris, M.; Lee, R.W.; Raubenheimer, D.; Simpson, S.J. Arthropod food webs become increasingly lipid-limited at higher trophic levels. Ecol. Lett. 2013, 16, 895–902. [Google Scholar] [CrossRef] [PubMed]




| Predator | Prey | Development Time (Days) | ||||
|---|---|---|---|---|---|---|
| Egg | Larva | Protonymph | Deutonymph | Total | ||
| N. b | T. u N1 | 1.600 ± 0.100 | 0.700 ± 0.065 | 1.400 ± 0.072 | 1.200 ± 0.118 | 4.900 ± 0.240 |
| S. t L1 | 1.750 ± 0.095 | 0.625 ± 0.082 | 2.375 ± 0.227 * | 2.375 ± 0.324 * | 7.125 ± 0.3988 * | |
| t | −0.974 | - | - | - | −5.085 | |
| U | - | 51.000 | 7.500 | 9.500 | - | |
| Sig. | 0.341 | 0.482 | <0.001 | <0.001 | <0.001 | |
| Predator | Prey | Development Time (Days) | ||||
|---|---|---|---|---|---|---|
| First instar larva | Second instar larva | Prepupa | Pupa | Total | ||
| S. t | T. u N1 | 1.767 ± 0.067 | 1.867 ± 0.133 | 0.867 ± 0.059 | 1.633 ± 0.059 | 6.133 ± 0. 192 |
| N. b E | 2.778 ± 0.147 * | 3.111 ± 0.505 * | 0.667 ± 0.083 | 1.611 ± 0.073 | 8.167 ± 0.514 * | |
| t | −7.130 | −2.381 | - | - | −3.708 | |
| U | - | - | 40.500 | 64.500 | - | |
| Sig. | <0.001 | 0.041 | 0.060 | 0.812 | 0.004 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Wang, M.; Nima, Y.; Feng, X.; Li, G.; Yang, Y.; Li, Y.; Liu, H. Intraguild Prey Served as Alternative Prey for Intraguild Predators in a Reciprocal Predator Guild between Neoseiulus barkeri and Scolothrips takahashii. Insects 2023, 14, 561. https://doi.org/10.3390/insects14060561
Liu M, Wang M, Nima Y, Feng X, Li G, Yang Y, Li Y, Liu H. Intraguild Prey Served as Alternative Prey for Intraguild Predators in a Reciprocal Predator Guild between Neoseiulus barkeri and Scolothrips takahashii. Insects. 2023; 14(6):561. https://doi.org/10.3390/insects14060561
Chicago/Turabian StyleLiu, Mingxiu, Mian Wang, Yuzhen Nima, Xiaotian Feng, Guangyun Li, Yi Yang, Yaying Li, and Huai Liu. 2023. "Intraguild Prey Served as Alternative Prey for Intraguild Predators in a Reciprocal Predator Guild between Neoseiulus barkeri and Scolothrips takahashii" Insects 14, no. 6: 561. https://doi.org/10.3390/insects14060561