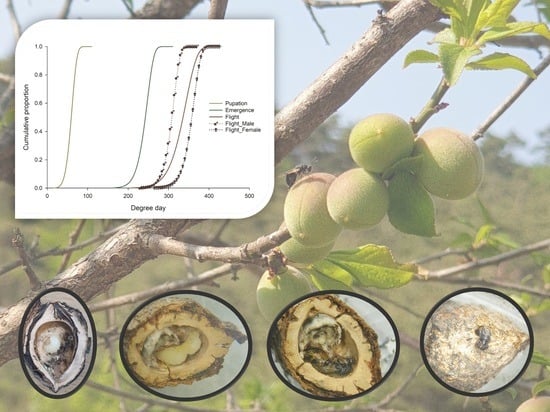
Temperature-Dependent Development of the Post-Diapause Periods of the Apricot Seed Wasp Eurytoma maslovskii (Hymenoptera: Eurytomidae): An Implication for Spring Emergence Prediction Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Source
2.2. Temperature-Dependent Development Experiment
2.3. Selection of Model for Describing the Development Rate
- (1)
- The model should comprise the desired statistical parameters to measure its standing as the model with the best fit. Here, we used the Akaike information criterion (AIC) (Equation (1)) and its corrected value (AICc) (Equation (2)) [20] for small sample size analysis to evaluate the goodness of fit of selected models.
- (2)
- The models should describe the observed data accurately and have parameters that allow estimates of biological significance, including lower temperature threshold (LT), optimal temperature (Topt), upper temperature threshold (UT), and total effective thermal constant (DD).
| Type | Model | Equation | Commentary | Reference |
|---|---|---|---|---|
| Linear | Ordinary | a and b are the slope and intercept of the linear regression, respectively. | [23] | |
| Ikemoto | D is the developmental duration at temperature T; K is thermal constant (degree day) and Tmin is the lower temperature threshold. | [24] | ||
| Non-linear | Longan-6 | ψ is the developmental rate at some base temperature above the developmental threshold, ρ is a constant defining the rate increase to the optimal temperature, Tmax is the lethal temperature threshold, and ΔT is the temperature range over which physiological breakdown becomes the overriding influence. | [25] | |
| Briere 1 | a is an empirical constant, Tmin is the lower temperature threshold, and Tmax is the lethal temperature threshold. | [26] | ||
| Briere 2 | a; Tmin; and Tmax are as in Birere-1, and d is an empirical constant. | [26] | ||
| Lactin 1 | ρ, Tmax, and ΔT are the same as in Logan-6. | [27] | ||
| Lactin 2 | ρ; Tmax; and ΔT are the same as in the Logan-6 model, and λ allows the curve to intersect the abscissa at suboptimal temperatures. | [27] | ||
| Inverse second-order polynomial | a, b, and c are empirical constants. | [13] | ||
| Third-order polynomial | a, b, c, and d are empirical constants. | [28] | ||
| Simplified beta type | ρ, α, and β are empirical constants. | [29] |
2.4. Distribution Model of Eurytoma maslovskii Post-Diapause
2.5. Model Validation and Field Monitoring
2.6. Data Analysis
3. Results
3.1. Temperature-Dependent Development
3.2. Selection of Models for Describing the Development Rate
3.3. Estimation of Model Parameters
3.4. Distribution Model
3.5. Field Monitoring and Model Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Model Type | Model Name | Temperature Range | p | Post-Diapause Larva | Pupa | Total Post-Diapause until Adult Emergence | Total Post-Diapause until Adult Flight | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adj. r2 | AIC | AICc | wi | Rank | Adj. r2 | AIC | AICc | wi | Rank | Adj. r2 | AIC | AICc | wi | Rank | AIC | AICc | wi | Rank | ||||
| Linear | Ordinary | 14.5–27.0 | 2 | 0.981 | −48.983 | −42.983 | 0.304 | 2 | 0.979 | −53.283 | −47.283 | 0.004 | 2 | 0.987 | −58.645 | −52.645 | 0.006 | 2 | −72.881 | −66.881 | 0.723 | 1 |
| Ordinary | 14.5–30.2 | 2 | 0.969 | −48.639 | −44.639 | 0.696 | 1 | 0.976 | −62.346 | −58.346 | 0.996 | 1 | 0.979 | −66.817 | −62.817 | 0.994 | 1 | −68.963 | −64.963 | 0.277 | 2 | |
| Ikemoto | 14.5–27.0 | 2 | 1.000 | −27.246 | −21.246 | 0.000 | 4 | 0.987 | 21.821 | 27.821 | 0.000 | 3 | 0.989 | 23.608 | 29.608 | 0.000 | 3 | 15.930 | 21.930 | 0.000 | 3 | |
| Ikemoto | 14.5–30.2 | 2 | 1.000 | −34.146 | −30.146 | 0.000 | 3 | 0.984 | 26.454 | 30.454 | 0.000 | 4 | 0.986 | 29.207 | 33.207 | 0.000 | 4 | 36.539 | 40.539 | 0.000 | 4 | |
| Non-linear | Longan_6 | 14.5–34.3 | 4 | 0.998 | −73.058 | −53.058 | 0.158 | 2 | 0.990 | −74.906 | −54.906 | 0.010 | 3 | 0.996 | −84.518 | −64.518 | 0.050 | 2 | −92.808 | −72.808 | 0.224 | 2 |
| Briere_1 | 14.5–34.3 | 3 | 0.942 | −52.261 | −44.261 | 0.002 | 3 | 0.921 | −64.403 | −56.403 | 0.021 | 2 | 0.932 | −72.094 | −64.094 | 0.041 | 3 | −76.760 | −68.760 | 0.030 | 3 | |
| Briere_2 | 14.5–34.3 | 4 | 0.947 | −48.782 | −28.782 | 0.000 | 6 | 0.947 | −62.403 | −42.403 | 0.000 | 6 | 0.885 | −65.242 | −45.242 | 0.000 | 6 | −76.150 | −56.150 | 0.000 | 5 | |
| Lactin_1 | 14.5–34.3 | 3 | 0.995 | −64.403 | −56.403 | 0.839 | 1 | 0.983 | −72.094 | −64.094 | 0.962 | 1 | 0.989 | −78.305 | −70.305 | 0.908 | 1 | −51.861 | −43.861 | 0.000 | 8 | |
| Lactin_2 | 14.5–34.3 | 4 | 0.992 | −62.403 | −42.403 | 0.001 | 4 | 0.989 | −74.001 | −54.001 | 0.006 | 4 | 0.989 | −74.001 | −54.001 | 0.000 | 5 | −95.208 | −75.208 | 0.743 | 1 | |
| Inverse second-order polynomial | 14.5–34.3 | 3 | 0.538 | −33.614 | −25.614 | 0.000 | 8 | 0.489 | −47.009 | −39.009 | 0.000 | 7 | 0.489 | −47.009 | −39.009 | 0.000 | 8 | −55.976 | −47.976 | 0.000 | 7 | |
| Third-order polynomial | 14.5–34.3 | 4 | 0.942 | −48.145 | −28.145 | 0.000 | 7 | 0.884 | −57.551 | −37.551 | 0.000 | 8 | 0.903 | −62.403 | −42.403 | 0.000 | 7 | −70.094 | −50.094 | 0.000 | 6 | |
| Simplified beta type | 14.5–34.3 | 3 | 0.943 | −48.285 | −40.285 | 0.000 | 5 | 0.919 | −59.551 | −51.551 | 0.002 | 5 | 0.928 | −64.403 | −56.403 | 0.001 | 4 | −72.094 | −64.094 | 0.003 | 4 | |
| Model | Parameter | Larva | Pupa | Entire Period until Adult Emergence | Entire Period until Adult Exit | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female | Total | Male | Female | ||||
| Ordinary (linear) | a | 0.0151 ± 0.0012 | 0.00553 ± 0.000383 | 0.00405 ± 0.000264 | 0.00428 ± 0.000377 | 0.00390 ± 0.000249 | 0.00297 ± 0.00006 | 0.00321 ± 0.00004 | 0.00277 ± 0.00007 |
| b | −0.123 ± 0.0279 | −0.0452 ± 0.00890 | −0.0331 ± 0.00613 | −0.0354 ± 0.00874 | −0.0313 ± 0.00578 | −0.0217 ± 0.00133 | −0.0242 ± 0.00078 | −0.0196 ± 0.00145 | |
| LT (°C) | 8.1 | 8.2 | 8.2 | 8.3 | 8.0 | 7.3 | 7.5 | 7.1 | |
| K (degree-day) | 66.2 | 180.8 | 246.9 | 233.6 | 256.4 | 336.7 | 311.5 | 361.0 | |
| r2 | 0.975 | 0.981 | 0.983 | 0.970 | 0.984 | 0.999 | 0.999 | 0.998 | |
| Longan 6 (non-linear) | ᴪ | 0.0198 ± 0.0030 | 0.0088 ± 0.0026 | 0.0061 ± 0.0013 | 0.0055 ± 0.0016 | 0.0061 ± 0.0012 | 0.0059 0.0009 | 0.0056 ± 0.0010 | 0.0059 ± 0.0006 |
| ρ | 0.1115 ± 0.0090 | 0.0989 ± 0.0153 | 0.1021 ± 0.0114 | 0.1111 ± 0.0173 | 0.1007 ± 0.0109 | 0.0928 0.0087 | 0.1000 ± 0.0112 | 0.0909 ± 0.0053 | |
| Tmax | 34.3002 ± 0.0262 | 34.3000 ± 0.0574 | 34.3001 ± 0.0406 | 34.2989 ± 0.0511 | 34.3000 ± 0.0396 | 34.3008 0.0346 | 34.3014 ± 0.0380 | 34.3001 ± 0.0216 | |
| ΔT | 3.2438 ± 0.3315 | 2.7001 ± 0.5920 | 2.8418 ± 0.4365 | 3.1763 ± 0.6327 | 2.8156 ± 0.4204 | 3.0952 0.3675 | 3.4342 ± 0.4633 | 3.0839 ± 0.2263 | |
| r2 | 0.9992 | 0.9952 | 0.9977 | 0.9968 | 0.9978 | 0.9986 | 0.9985 | 0.9995 | |
| Briere 1 (non-linear) | A | 0.0003 ± 0.00005 | 0.00009 ± 0.00002 | 0.00007 ± 0.00002 | 0.00007 ± 0.00002 | 0.00006 ± 0.00002 | 0.0005 ± 0.00009 | 0.0005 ± 0.00007 | 0.0004 ± 0.00009 |
| Tmax | 34.3000 ± 1.4549 | 34.3000 ± 1.7050 | 34.3000 ± 1.5783 | 34.3000 ± 1.6246 | 34.3000 ± 1.5920 | 34.3000 ± 1.3359 | 34.3000 ± 0.9905 | 34.3000 ± 1.4014 | |
| Tmin | 10.4962 ± 1.7161 | 10.4981 ± 2.0106 | 10.5015 ± 1.8602 | 10.5678 ± 1.8968 | 10.4321 ± 1.8952 | 9.2334 ± 1.8743 | 8.5034 ± 1.5268 | 9.0640 ± 2.0103 | |
| r2 | 0.9613 | 0.9471 | 0.9545 | 0.9526 | 0.9533 | 0.9620 | 0.9797 | 0.9573 | |
| Lactin 1 (non-linear) | ρ | 0.1800 ± 0.0037 | 0.1812 ± 0.0068 | 0.1809 ± 0.0055 | 0.1813 ± 0.0050 | 0.1802 ± 0.0056 | (not fit observed data) | ||
| Tmax | 34.3085 ± 0.0508 | 34.3136 ± 0.0933 | 34.3122 ± 0.0755 | 34.3072 ± 0.0685 | 34.3126 ± 0.0771 | ||||
| ΔT | 5.5457 ± 0.1118 | 5.5156 ± 0.2059 | 5.5255 ± 0.1668 | 5.5126 ± 0.1515 | 5.5455 ± 0.1703 | ||||
| r2 | 0.9967 | 0.9888 | 0.9927 | 0.9940 | 0.9924 | ||||
| Lactin 2 (non-linear) | Ρ | 0.0131 ± 0.0008 | 0.0053 ± 0.0004 | 0.0041 ± 0.0003 | 0.0044 ± 0.0004 | 0.0039 ± 0.0003 | 0.0029 ± 0.0001 | 0.0031 ± 0.00009 | 0.0027 ± 0.0001 |
| Tmax | 36.6099 ± 0.4056 | 37.3780 ± 0.8020 | 37.8752 ± 0.7284 | 38.1434 ± 1.0169 | 37.9211 ± 0.7447 | 39.1740 ± 0.3890 | 39.4152 ± 0.2679 | 39.3332 ± 0.5015 | |
| ΔT | 1.8017 ± 0.3330 | 1.4869 ± 0.4044 | 1.5322 ± 0.3267 | 1.6873± 0.4719 | 1.5320 ± 0.3296 | 1.8680 ± 0.1591 | 2.0142 ± 0.1137 | 1.8902 ± 0.2012 | |
| λ | −1.1184 ± 0.0227 | −1.0473 ± 0.0100 | −1.0364 ± 0.0062 | −1.0407 ± 0.0100 | −1.0346 ± 0.0060 | −1.0215 ± 0.0025 | −1.0246 ± 0.0019 | −1.0194 ± 0.0029 | |
| r2 | 0.9958 | 0.9946 | 0.9963 | 0.9917 | 0.9962 | 0.9990 | 0.9995 | 0.9984 | |

References
- Zerova, M.D.; Fursov, V.N. The Palaearctic species of Eurytoma (Hymenoptera: Eurytomidae) developing in stone fruits (Rosaceae: Prunoideae). Bull. Entomol. Res. 1991, 81, 209–219. [Google Scholar] [CrossRef]
- Wang, W.S. A new pest in apricot fruit—Eurytoma maslovskii. Plant Quar. 2000, 14, 94–95. [Google Scholar] [CrossRef]
- Lee, S.-M.; Kim, S.-J.; Yang, C.Y.; Shin, J.-S.; Hong, K.-J. Host plant, occurrence, and oviposition of the eurytomid wasp Eurytoma maslovskii in Korea. Korean J. Appl. Entomol. 2014, 53, 381–389. [Google Scholar] [CrossRef]
- Tachikawa, T. Eurytoma maslovskii Nikolskaja newly discovered from Korea (Hymenoptera: Chalcidoidea–Eurytomidae). Trans. Shikoku Ent. Soc. 1979, 14, 181–183. [Google Scholar]
- Choi, D.-S.; Ko, S.-J.; Ma, K.-C.; Kim, H.-J.; Kim, D.-I.; Kim, H.-W. Damage, occurrence, and optimal control period of Eurytoma maslovskii affecting Japanese apricot (Prunus mume) fruits in Jeonnam province. Korean J. Appl. Entomol. 2015, 54, 191–197. [Google Scholar] [CrossRef]
- Tang, G.; Niu, J.; Liu, Y.; Zhou, X.; Lu, R.; Zheng, C.; Guan, W. Bionomics of Eurytoma maslovskii Nikolskaya. For. Pest Dis. 1999, 18, 5–7. [Google Scholar]
- Zhu, Z.J.; Tu, Y.H.; Pan, G.L.; Cao, Y.S.; Shu, J. Study on biological properties of Eurytomam maslovskii. J. Zhejiang For. Sci. Technol. 2010, 30, 38–41. [Google Scholar]
- Zhang, K.P.; Wu, H.B.; Gong, Q.; Sun, R.H. The emergence rhythm of Eurytoma maslovskii Nikolskaya adults in field and its influencing factors. J. Henan Agric. Sci. 2017, 46, 91–94. [Google Scholar]
- Wang, X.N.; Zhao, K.; Chu, X.M.; Li, P.; Bao, Z.F.; Duan, C.H. Emergence dynamics of Eurytoma maslovskii and the forecasting model. For. Res. 2005, 18, 95–97. [Google Scholar]
- Mohammod, A.; Kim, B.S.; Park, S.J.; Kim,, H.J. Development of a plum (Japanese apricot) seed crusher to control harmful larvae (Eurytoma maslovskii) in Plum Orchard. J. Agric. Biol. Sci. 2019, 53, 153–162. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Song, J.-H.; Choi, J.-H.; Choi, J.-J.; Kim, M.-S. Eco-friendly materials selection and control timing to Eurytoma maslovskii in Japanese Apricot. Korean J. Org. Agric. 2016, 24, 123–130. [Google Scholar] [CrossRef]
- Yang, C.Y.; Mori, K.; Kim, J.; Kwon, K.B. Identification and field bioassays of the sex pheromone of Eurytoma maslovskii (Hymenoptera: Eurytomidae). Sci. Rep. 2020, 10, 10281. [Google Scholar] [CrossRef] [PubMed]
- Damos, P.; Savopoulou-Soultani, M. Temperature-driven models for insect development and vital thermal requirements. Psyche 2012, 2012, 123405. [Google Scholar] [CrossRef]
- Geng, S.; Jung, C. Temperature-dependent development of overwintering pupae of Phyllonorycter ringoniella and its spring emergence model. J. Asia Pac. Entomol. 2018, 21, 829–835. [Google Scholar] [CrossRef]
- Kim, D.-S.; Lee, J.-H. A population model for the peach fruit moth, Carposina sasakii Matsumura (Lepidoptera: Carposinidae), in a Korean orchard system. Ecol. Model. 2010, 221, 268–280. [Google Scholar] [CrossRef]
- Park, C.G.; Kim, D.S.; Lee, S.M.; Moon, Y.S.; Chung, Y.J.; Kim, D.-S. A forecasting model for the adult emergence of overwintered Monochamus alternatus (Coleoptera: Cerambycidae) larvae based on degree-days in Korea. Appl. Entomol. Zool. 2014, 49, 35–42. [Google Scholar] [CrossRef]
- Sridhar, V.; Reddy, P.V.R. Use of degree days and plant phenology: A reliable tool for predicting insect pest activity under climate change conditions. In Climate-Resilient Horticulture: Adaptation and Mitigation Strategies; Singh, H.C.P., Rao, N.K.S., Shivashankar, K.S., Eds.; Springer: New Delhi, India, 2013; pp. 287–294. [Google Scholar] [CrossRef]
- Knight, A.L. Adjusting the phenology model of codling moth (Lepidoptera: Tortricidae) in Washington state apple orchards. Environ. Entomol. 2014, 36, 1485–1493. [Google Scholar] [CrossRef]
- Baskerville, G.L.; Emin, P. Rapid estimation of heat accumulation from maximum and minimum temperatures. Ecology 1969, 50, 514–517. [Google Scholar] [CrossRef]
- Akaike, H. Information theory and an extension of the maximum likelihood principle. In Selected Papers of Hirotugu Akaike; Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Wagenmakers, E.-J.; Farrell, S. AIC model selection using Akaike weights. Psychon. Bull. Rev. 2004, 11, 192–196. [Google Scholar] [CrossRef]
- Campbell, A.; Frazer, B.D.; Gilbert, N.; Gutierrez, A.P.; Mackauer, M. Temperature requirements of some aphids and their parasites. J. Appl. Ecol. 1974, 11, 431–438. [Google Scholar] [CrossRef]
- Ikemoto, T.; Takai, K. A new linearized formula for the law of total effective temperature and the evaluation of line-fitting methods with both variables subject to error. Environ. Entomol. 2000, 29, 671–682. [Google Scholar] [CrossRef]
- Logan, J.A.; Wollkind, D.J.; Hoyt, S.C.; Tanigoshi, L.K. An analytic model for description of temperature dependent rate phenomena in arthropods. Environ. Entomol. 1976, 5, 1133–1140. [Google Scholar] [CrossRef]
- Briere, J.-F.; Pracros, P.; Le Roux, A.-Y.; Pierre, J.-S. A novel rate model of temperature-dependent development for arthropods. Environ. Entomol. 1999, 28, 22–29. [Google Scholar] [CrossRef]
- Lactin, D.J.; Holliday, N.J.; Johnson, D.L.; Craigen, R. Improved rate model of temperature-dependent development by arthropods. Environ. Entomol. 1995, 24, 68–75. [Google Scholar] [CrossRef]
- Harcourt, D.G.; Yee, J.M. Polynomial algorithm for predicting the duration of insect life stages. Environ. Entomol. 1982, 11, 581–584. [Google Scholar] [CrossRef]
- Damos, P.T.; Savopoulou-Soultani, M. Temperature-dependent bionomics and modeling of Anarsia lineatella (Lepidoptera: Gelechiidae) in the laboratory. J. Econ. Entomol. 2008, 101, 1557–1567. [Google Scholar] [CrossRef]
- Wagner, T.; Wu, H.; Sharpe, P.; Coulson, R. Modeling distributions of insect development time: A literature review and application of the Weibull function. Ann. Entomol. Soc. Am. 1984, 77, 475–483. [Google Scholar] [CrossRef]
- Grubbs, F.E. Sample criteria for testing outlying observations. Ann. Math. Stat. 1950, 21, 27–58. [Google Scholar] [CrossRef]
- Marquardt, D.W. An algorithm for least-squares estimation of nonlinear parameters. SIAM J. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Danks, H.V. Long life cycles in insects. Can. Entomol. 1992, 124, 167–187. [Google Scholar] [CrossRef]
- Smith, D.N. Prolonged larval development in Buprestis aurulenta L. (Coleoptera: Buprestidae). A Review with new cases. Can. Entomol. 1962, 94, 586–593. [Google Scholar] [CrossRef]
- Camillo, E. A solitary mud-daubing wasp, Brachymenes dysmenes (Hymenoptera: Vespidae) from Brazil with evidence of a life-cycle polyphenism. Rev. Biol. Trop. 1999, 47, 949–958. [Google Scholar]
- Xu, R.; Chen, Y.-H.; Xia, J.-F.; Zeng, T.-X.; Li, Y.-H.; Zhu, J.-Y. Metabolic dynamics across prolonged diapause development in larvae of the sawfly, Cephalcia chuxiongica (Hymenoptera: Pamphiliidae). J. Asia Pac. Entomol. 2021, 24, 1–6. [Google Scholar] [CrossRef]
- Graf, B.; Höhn, H.; Höpli, H.U. The apple sawfly, Hoplocampa testudinea: A temperature driven model for spring emergence of adults. Entomol. Exp. Appl. 1996, 78, 301–307. [Google Scholar] [CrossRef]


| Temperature(°C) | n | Duration (Days) | Percentage (%) | Sex Ratio (M:F) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Larva | Pupa | Total | Male | Female a | Prolong b | Pupation | Emergence | |||
| 14.5 | 49 | 10.0 ± 0.20 a | 29.1 ± 0.19 a | 39.1 ± 0.34 a | 36.8 ± 0.44 a | 40.0 ± 0.32 a,** | 4.1 | 95.9 | 91.8 | 1:2.8 |
| 18.8 | 47 | 6.8 ± 0.21 b | 17.0 ± 0.15 b | 23.8 ± 0.29 b | 23.3 ± 0.44 b | 24.4 ± 0.37 b, ns | 4.3 | 89.4 | 85.1 | 1:1.0 |
| 21.3 | 47 | 5.0 ± 0.16 c | 13.7 ± 0.16 c | 18.6 ± 0.22 c | 18.0 ± 0.34 c | 19.3 ± 0.19 c,** | 2.1 | 91.5 | 89.4 | 1:1.0 |
| 24.0 | 47 | 4.0 ± 0.09 d | 11.9 ± 0.10 d | 15.8 ± 0.14 d | 15.1 ± 0.16 d | 16.3 ± 0.14 d,** | 2.1 | 93.6 | 93.6 | 1:1.4 |
| 27.0 | 47 | 3.3 ± 0.07 e | 8.9 ± 0.11 e | 12.2 ± 0.14 e | 11.3 ± 0.14 e | 12.6 ± 0.13 e,** | 4.3 | 85.1 | 83.0 | 1:2.3 |
| 30.2 | 46 | 3.1 ± 0.14 e | 8.5 ± 0.10 e | 11.7 ± 0.19 e | 11.3 ± 0.24 e | 12.1 ± 0.27 e,* | 21.7 | 60.9 | 58.7 | 1:0.9 |
| 34.3 | 49 | - c | - | - | - | - | 100.0 | - | - | |
| p-value | <0.001 | <0.001 | <0.001 | |||||||
| Temperature(°C) | n | Duration (Days) | Percentage (%) | Post-Emergence d (Days) | Sex Ratio | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Male | Female a | Prolong b | Exit | Total | Male | Female | (M:F) | ||
| 14.5 | 81 | 46.3 ± 0.32 a | 44.7 ± 0.55 a | 47.3 ± 0.33 a,** | 1.2 | 90.1 | 7.2 | 7.9 | 7.3 | 1:1.6 |
| 18.8 | 47 | 29.7 ± 0.33 b | 27.9 ± 0.22 b | 31.0 ± 0.34 b,** | 6.4 | 80.9 | 5.8 | 4.6 | 6.7 | 1:1.4 |
| 21.3 | 68 | 23.9 ± 0.26 c | 22.4 ± 0.21 c | 25.8 ± 0.18 c,** | 1.5 | 94.1 | 5.3 | 4.4 | 6.5 | 1:0.8 |
| 24.0 | 83 | 20.5 ± 0.17 d | 19.0 ± 0.17 d | 21.4 ± 0.14 d,** | 1.2 | 94.0 | 4.6 | 3.9 | 5.1 | 1:1.5 |
| 27.0 | 74 | 17.0 ± 0.17 e | 16.0 ± 0.20 e | 17.9 ± 0.14 e,** | 2.7 | 93.2 | 4.7 | 4.7 | 5.3 | 1:1.1 |
| 30.2 | 63 | 16.7 ± 0.24 e | 15.8 ± 0.25 e | 17.8 ± 0.31 e,** | 1.6 | 69.8 | 5.0 | 4.5 | 5.7 | 1:0.8 |
| 34.3 | 33 | - c | - | - | 87.9 | - | ||||
| p-value | <0.001 | <0.001 | <0.001 | |||||||
| Model | Model Parameter | r2 | |
|---|---|---|---|
| a | b | ||
| Pupation | 63.2202 ± 1.1350 | 6.0138 ± 0.9486 | 0.8849 |
| Adult emergence | 248.2907 ± 1.4045 | 15.8672 ± 1.8857 | 0.8928 |
| Adult exit | 344.9728 ± 0.8859 | 13.5357 ± 0.6402 | 0.9447 |
| - Male | 313.8498 ± 0.8076 | 21.1625 ± 1.7056 | 0.9316 |
| - Female | 363.6323 ± 1.0307 | 21.8888 ± 1.7971 | 0.9101 |
| Year | Location | Observed/Predicted Julian Date at Cumulative Point | Difference a | t-Value b | r2 c | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 10% | 30% | 50% | 70% | 90% | 3 Days | 5 Days | ||||
| 2015 | Sunchang | 116/111 | 117/114 | 120/116 | 121/117 | 122/119 | 3.8 ± 0.4 | 2.14 ns | -3.21 ns | 0.9691 |
| Yeongkwang | 116/119 | 117/121 | 119/123 | 120/124 | 121/127 | 4.2 ± 0.5 | 2.45 * | -1.63 ns | 0.9778 | |
| Suncheon | 112/114 | 114/117 | 115/119 | 117/120 | 124/122 | 2.8 ± 0.4 | −0.53 ns | −5.88 ns | 0.8917 | |
| Goheung | 112/112 | 114/115 | 115/117 | 120/119 | 124/120 | 1.6 ± 0.7 | −2.06 ns | −5.01 ns | 0.9222 | |
| Gwangyang | 112/110 | 115/113 | 117/115 | 121/116 | 124/117 | 3.6 ± 1.0 | 0.58 ns | −1.36 ns | 0.9493 | |
| 2016 | Sunchang | 112/113 | 116/116 | 120/117 | 121/119 | 122/121 | 1.4 ± 0.5 | −3.14 ns | −7.06 ns | 0.9500 |
| Yeongkwang | 110/117 | 114/121 | 119/123 | 121/124 | 124/126 | 4.6 ± 1.0 | 1.55 ns | −0.39 ns | 0.9850 | |
| Suncheon | 109/109 | 114/113 | 116/114 | 120/116 | 122/117 | 2.4 ± 0.9 | −0.65 ns | −2.8 ns | 0.9911 | |
| Goheung | 105/112 | 109/115 | 114/117 | 116/120 | 121/122 | 4.2 ± 1.1 | 1.12 ns | −0.75 ns | 0.9864 | |
| Gwangyang | 106/105 | 110/108 | 111/110 | 112/112 | 115/113 | 1.2 ± 0.4 | −4.81 ns | −10.16 ns | 0.9668 | |
| 2022 | Room | 093/094 | 095/096 | 096/097 | 096/097 | 097/098 | 1.0 ± 0.0 | - d | - | 1.0000 |
| Semi-field | 120/117 | 122/122 | 125/124 | 126/125 | 128/127 | 1.2 ± 0.5 | −3.67 ns | −7.76 ns | 0.9661 | |
| Suncheon | 108/108 | 111/112 | 113/113 | 114/114 | 117/116 | 0.4 ± 0.2 | −10.61 ns | −18.78 ns | 0.9828 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.N.; Lee, I.J.; Kim, H.J.; Hong, K.-J. Temperature-Dependent Development of the Post-Diapause Periods of the Apricot Seed Wasp Eurytoma maslovskii (Hymenoptera: Eurytomidae): An Implication for Spring Emergence Prediction Models. Insects 2022, 13, 722. https://doi.org/10.3390/insects13080722
Nguyen HN, Lee IJ, Kim HJ, Hong K-J. Temperature-Dependent Development of the Post-Diapause Periods of the Apricot Seed Wasp Eurytoma maslovskii (Hymenoptera: Eurytomidae): An Implication for Spring Emergence Prediction Models. Insects. 2022; 13(8):722. https://doi.org/10.3390/insects13080722
Chicago/Turabian StyleNguyen, Hai Nam, In Jun Lee, Hyuck Joo Kim, and Ki-Jeong Hong. 2022. "Temperature-Dependent Development of the Post-Diapause Periods of the Apricot Seed Wasp Eurytoma maslovskii (Hymenoptera: Eurytomidae): An Implication for Spring Emergence Prediction Models" Insects 13, no. 8: 722. https://doi.org/10.3390/insects13080722