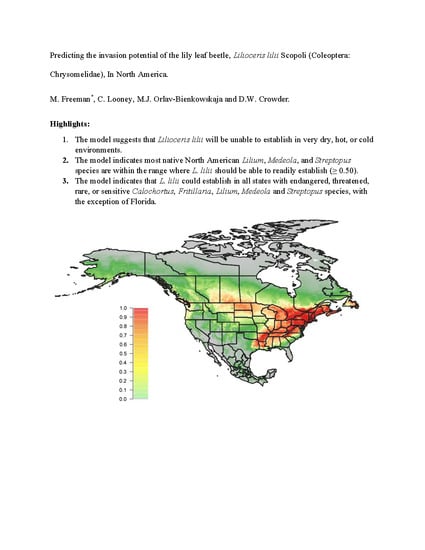
Predicting the Invasion Potential of the Lily Leaf Beetle, Lilioceris lilii Scopoli (Coleoptera: Chrysomelidae), in North America
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Occurrence Records
2.2. Bioclimatic Variables
2.3. Species Distribution Modelling in Maxent
2.4. Liliaceae Site Records
3. Results
3.1. Exploitation of Available and Occupied Bioclimatic Ranges
3.2. Predicted Global Distribution Based on All Known Occurrences
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walker, B.; Steffen, W. An overview of the implications of global change for natural and managed terrestrial ecosystems. Conserv. Ecol. 1997, 2, 2. [Google Scholar] [CrossRef]
- Mooney, H.A.; Cleland, E.E. The evolutionary impact of invasive species. Proc. Natl. Acad. Sci. USA 2001, 98, 5446–5451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, O.E.; Chapin, F.S., III; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Work, T.T.; McCullough, D.G.; Cavey, J.F.; Komsa, R. Arrival rate of nonindigenous insect species into the United States through foreign trade. Biol. Invasions 2005, 7, 323–332. [Google Scholar] [CrossRef]
- Perrings, C.; Dehnen-Schmutz, K.; Touza, J.; Williamson, M. How to manage biological invasions under globalization. Trends Ecol. Evol. 2005, 20, 212–215. [Google Scholar] [CrossRef]
- Poulin, R.; Paterson, R.A.; Townsend, C.R.; Tompkins, D.M.; Kelly, D.W. Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshw. Biol. 2011, 56, 676–688. [Google Scholar] [CrossRef]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Araujo, M.B.; Guisan, A. Five (or so) challenges for species distribution modelling. J. Biogeogr. 2006, 33, 1677–1688. [Google Scholar] [CrossRef]
- Jıménez-Valverde, A.; Peterson, A.T.; Sobéron, J.; Overton, J.M.; Aragon, P.; Lobo, J.M. Use of niche models in invasive species risk assessments. Biol. Invasions 2011, 13, 2785–2797. [Google Scholar] [CrossRef]
- Baxter, P.W.J.; Possingham, H.P. Optimizing search strategies for invasive pests: Learn before you leap. J. Appl. Ecol. 2011, 48, 86–95. [Google Scholar] [CrossRef]
- Rodríguez, J.; Brotons, L.; Bustamante, J.; Seoane, J. The application of predictive modelling of species distribution to biodiversity conservation. Divers. Distrib. 2007, 13, 243–251. [Google Scholar] [CrossRef]
- Richardson, D.M.; Whittaker, R.J. Conservation biogeography- foundations, concepts and challenges. Divers. Distrib. 2010, 16, 313–320. [Google Scholar] [CrossRef]
- Guisan, A.; Tingley, R.; Baumgartner, J.B.; Naujokaitis-Lewis, I.; Sutcliffe, P.R.; Tulloch, A.I.T.; Regan, T.J.; Brotons, L.; McDonald-Madden, E.; Mantyka-Pringle, C.; et al. Predicting species distributions for conservation decisions. Ecol. Lett. 2013, 16, 1424–1435. [Google Scholar] [CrossRef] [PubMed]
- Tewksbury, L. Introduction and Establishment of Three Parasitoids of the Lily Leaf Beetle, Lilioceris lilii, (Coleoptera: Chrysomelidae) in North America. Ph.D. Thesis, University of Rhode Island, Kingston, RI, USA, 2014. [Google Scholar]
- Salisbury, A. Impact, Host Range and Chemical Ecology of the Lily Beetle, Lilioceris lilii. Ph.D. Thesis, Imperial College London, London, UK, 2008. [Google Scholar]
- Say, T. Descriptions of new species of coleopterous insects inhabiting the United States. J. Proc. Acad. Nat. Sci. Phil. 1825, 5, 294. Available online: http://www.biodiversitylibrary.org/item/79357#page/316/mode/1up (accessed on 4 September 2019).
- Majka, C.G.; LeSage, L. Introduced leaf beetles of the Maritime Provinces, 5: The lily leaf beetle, Lilioceris lilii (Scopoli) (Coleoptera: Chrysomelidae). Proc. Entomol. Soc. Wash. 2008, 110, 186–195. [Google Scholar] [CrossRef]
- Lesage, L. Note sur la distribution présente et future du Criocére du lys, Lilioceris lilii (Scopoli) (Coleoptera: Chrysomelidae) dans l’est du Canada. Nat. Can. 1983, 110, 95–97. [Google Scholar]
- Ernst, C.; Cappuccino, N.; Thor, A. Potential novel hosts for the lily leaf beetle Lilioceris lilii Scopoli (Coleoptera: Chrysomelidae) in eastern North America. Ecol. Entomol. 2007, 32, 45–52. [Google Scholar] [CrossRef]
- Cappuccino, N. Lily Leaf Beetle Tracker. Available online: http://lilybeetletracker.weebly.com (accessed on 30 October 2019).
- Murray, T.; Looney, C.; LaGasa, E.; Collman, S. Distribution of two invasive leaf beetles, Pyrrhalta viburni (Paykull) and Lilioceris lilii (Coleoptera Chrysomelidae), in Washington State. Coleopt. Bull. 2016, 70, 368–371. [Google Scholar] [CrossRef]
- WSDA. Lily Leaf Beetle Report Form. Available online: https://wsda.maps.arcgis.com/apps/GeoForm/index.html?appid=51d4e1ae6f9841fea29396d942d67434 (accessed on 30 October 2019).
- Bouchard, A.; McNeil, J.N.; Brodeur, J. Invasion of American native lily populations by an alien beetle. Biol. Invasions 2008, 10, 1365–1372. [Google Scholar] [CrossRef]
- Blackman, C.K.; Cappuccino, N.; Mason, P.G. First record of Lilioceris lilii (Scopoli) (Coleoptera: Chrysomelidae) on Lilium michiganense Farwell and confirmation of its association with Streptopus lanceolatus (Aiton) Reveal (Liliaceae). Coleopt. Bull. 2016, 70, 482–484. [Google Scholar] [CrossRef]
- Freeman, M.; Looney, C.; Crowder, D.W. Lilioceris lilii (Coleoptera: Chrysomelidae) oviposition behavior and development on Liliaceae (Liliales) native to the Pacific Northwest. (manuscript in preparation).
- USDA, NRCS. The Plants Database. Available online: http://www.plants.usda.gov/ (accessed on 15 July 2019).
- Gold, M.S.; Casagrande, R.A.; Tewksbury, L.; Livingston, S.B.; Kenis, M. European parasitoids of Lilioceris lilii (Coleoptera: Chrysomelidae). Can. Entomol. 2001, 133, 671–674. [Google Scholar] [CrossRef] [Green Version]
- Kenis, M.; Haye, T.; Casagrande, R.A.; Gold, M.S.; Tewksbury, L.A. Selection and importation of European parasitoids for the biological control of the lily leaf beetle in North America, and prospects for control in Europe. In Proceedings of the First International Symposium of Biological Control of Arthropods, Honolulu, HI, USA, 14–18 January 2002. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Dudik, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open source release of Maxent. Ecography 2017, 40, 887–898. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M.; Schapirem, R.E. Maxent Software for Modeling Species Niches and Distributions (Version 3.4.1); American Museum of Natural History: New York, NY, USA, 2018. [Google Scholar]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudik, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129. [Google Scholar] [CrossRef] [Green Version]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of Maxent for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Cappuccino, N.; Carleton University, Ottawa, ON, Canada. Personal Communication, 2018.
- Yu, P.; Lu, W.; Casagrande, R. Lilioceris lilii (Scopoli) occurs in China (Coleoptera: Chrysomelidae). Coleopt. Bull. 2001, 55, 65–66. [Google Scholar] [CrossRef]
- Brzica, M. Bio-Ecological Research of Lily Leaf Beetle Lilioceris lilii Scopoli (Coleoptera: Chrysomelidae) in Bosnia and Herzegovina. Entomol. Hell. 2011, 20, 55–67. [Google Scholar] [CrossRef]
- Dieni, A.; Brodeur, J.; Turgeon, J. Reconstructing the invasion history of the lily leaf beetle, Lilioceris lilii, in North America. Biol. Invasions 2016, 18, 31–44. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M. Dynamics of the range of lily leaf beetle (Lilioceris lilii, Chrysomelidae, Coleoptera) indicates its invasion form Asia to Europe in the 16–17th century. Russ. J. Biol. Invasions 2013, 4, 93–104. [Google Scholar] [CrossRef]
- Orlova-Bienkowskaja, M.J. Inventory of Alien Beetles of European Russia; Severtsov Russian Academy of Sciences: Moscow, Russia, 2019. [Google Scholar]
- Bezděk, J.; Scmitt, M. Catalogue of Palaearctic Coleoptera vol. 6, Corrigenda et Addenda. Entomol. Blätter Coleopt. 2017, 133, 113–135. [Google Scholar]
- Phillips, S.J.; Dudík, M.; Elith, J.; Graham, C.H.; Lehmann, A.; Leathwick, J.; Ferrier, S. Sample selection bias and presence-only distribution models: Implications for background and pseudo-absence data. Ecol. Appl. 2009, 19, 181–197. [Google Scholar] [CrossRef] [Green Version]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to Maxent for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Fourcade, Y.; Engler, J.O.; Rödder, D.; Secondi, J. Mapping Species Distributions with Maxent Using a Geographically Biased Sample of Presence Data: A Performance Assessment of Methods for Correcting Sampling Bias. PLoS ONE 2014, 9, e97122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-km resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2012, 36, 027–046. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The Effects of Sampling Bias and Model Complexity on the Predictive Performance of Maxent Species Distribution Models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Pradhan, P. Strengthening Maxent modelling through screening of redundant explanatory Bioclimatic Variables with Variance Inflation Factor analysis. Researcher 2016, 8, 29–34. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Broennimann, O.; Guisan, A. Predicting current and future biological invasions: Both native and invaded ranges matter. Biol. Lett. 2008, 4, 585–589. [Google Scholar] [CrossRef]
- Beaumont, L.J.; Gallagher, R.V.; Thuiller, W.; Downey, P.O.; Leishman, M.R.; Hughes, L. Different climate envelopes among invasive populations may lead to underestimations of current and future biological invasions. Divers. Distrib. 2009, 15, 409–420. [Google Scholar] [CrossRef]
- Medley, K.A. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Glob. Ecol. Biogeogr. 2010, 19, 122–133. [Google Scholar] [CrossRef]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Moreno, P.; Diez, J.M.; Richardson, D.M.; Vilà, M. Beyond climate: Disturbance niche shifts in invasive species. Glob. Ecol. Biogeogr. 2015, 24, 360–370. [Google Scholar] [CrossRef]
- Chapman, D.S.; Scalone, R.; Štefanić, E.; Bullock, J.M. Mechanistic species distribution modeling reveals a niche shift during invasion. Ecology 2017, 98, 1671–1680. [Google Scholar] [CrossRef] [Green Version]
- Wiens, J.J.; Graham, C.H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef] [Green Version]
- Pearman, P.B.; Guisan, A.; Broennimann, O.; Randin, C.F. Niche dynamics in space and time. Trends Ecol. Evol. 2008, 23, 149–158. [Google Scholar] [CrossRef]
- Pearce, J.; Ferrier, S. An evaluation of alternative algorithms for fitting species distribution models using logistic regression. Ecol. Model. 2000, 128, 128–147. [Google Scholar] [CrossRef]
- SERNEC Data Portal. Available online: http//:sernecportal.org/portal/index.php (accessed on 10 July 2019).
- Tewksbury, L.; Casagrande, R.A.; Cappuccino, N.; Kenis, M. Establishment of Parasitoids of the Lily Leaf Beetle (Coleoptera: Chrysomelidae) in North America. Environ. Entomol. 2017, 46, 226–236. [Google Scholar] [CrossRef]
- Tewksbury, L.; Gold, M.S.; Casagrande, R.A.; Kenis, M. Establishment in North America of Tetrastichus setifer Thomson (Hymenoptera: Eulophidae), a parasitoid of Lilioceris lilii (Coleoptera: Chrysomelidae). In Proceedings of the 2nd International Symposium on Biological control of Arthropods, Davos, Switzerland, 12–16 September 2005. [Google Scholar]
- Jeschke, J.M.; Strayer, D.L. Usefulness of Bioclimatic Models for Studying Climate Change and Invasive Species. Ann. N. Y. Acad. Sci. 2008, 1134, 1–24. [Google Scholar] [CrossRef]
- Haye, T.; Kenis, M. Biology of Lilioceris spp. (Coleoptera: Chrysomelidae) and their parasitoids in Europe. Biol. Control. 2004, 29, 399–408. [Google Scholar] [CrossRef]
- Peterson, T.A.; Papes, M.; Kluza, D.A. Predicting the potential invasive distributions of four alien plant species in North America. Weed Sci. 2003, 51, 863–868. [Google Scholar] [CrossRef]
- White, R. A Revision of the Subfamily Criocerinae (Chrysomelidae) of North America North of Mexico. In USDA-ARS Technical Bulletin 1805; United States Department of Agriculture Agricultural Research Service: Washington, DC, USA, 1993. [Google Scholar]
- Rushydromet. Second Roshhydrmet Assessment Report on Climate Change and Its Consequences in Russian Federation; Federal Service for Hydrometeorology and Environmental Monitoring: Moscow, Russia, 2015.
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M.; Kitoh, A.; Knutti, R.; Murphy, J.M.; Noda, A.; et al. Global Climate Projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hickling, R.; Roy, D.B.; Hill, J.K.; Fox, R.; Thomas, C.D. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Chang. Biol. 2006, 12, 450–455. [Google Scholar] [CrossRef]
- Deutsch, C.A.; Tewksbury, J.J.; Huey, R.B.; Sheldon, K.S.; Ghalambor, C.K.; Haak, D.C.; Martin, P.R. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl. Acad. Sci. USA 2008, 15, 6672–6688. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.D. Climate, climate change and range boundaries. Divers. Distrib. 2010, 16, 488–495. [Google Scholar] [CrossRef]
- Chen, C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Mason, S.C.; Palmer, G.; Fox, R.; Gillings, S.; Hill, J.K.; Thomas, C.D.; Oliver, T.H. Geographical range margins of many taxonomic groups continue to shift polewards. Biol. J. Linnaean Soc. 2015, 115, 586–597. [Google Scholar] [CrossRef]
- Battisti, A.; Stastny, M.; Netherer, S.; Robinet, C.; Schopf, A.; Roques, A.; Larsson, S. Expansion of geographic range in the pine processionary moth caused by increased winter temperatures. Ecol. Appl. 2005, 15, 2084–2096. [Google Scholar] [CrossRef]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukát, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 23, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Robinet, C.; Roques, A. Direct impacts of recent climate warming on insect populations. Integr. Zool. 2010, 5, 132–142. [Google Scholar] [CrossRef]
- Van der Veken, S.; Harmy, M.; Vellend, M.; Knapen, A.; Verheyen, K. Garden plants get a head start on climate change. Front. Ecol. Environ. 2008, 6, 212–216. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freeman, M.; Looney, C.; Orlova-Bienkowskaja, M.J.; Crowder, D.W. Predicting the Invasion Potential of the Lily Leaf Beetle, Lilioceris lilii Scopoli (Coleoptera: Chrysomelidae), in North America. Insects 2020, 11, 560. https://doi.org/10.3390/insects11090560
Freeman M, Looney C, Orlova-Bienkowskaja MJ, Crowder DW. Predicting the Invasion Potential of the Lily Leaf Beetle, Lilioceris lilii Scopoli (Coleoptera: Chrysomelidae), in North America. Insects. 2020; 11(9):560. https://doi.org/10.3390/insects11090560
Chicago/Turabian StyleFreeman, Maggie, Chris Looney, Marina J. Orlova-Bienkowskaja, and David W. Crowder. 2020. "Predicting the Invasion Potential of the Lily Leaf Beetle, Lilioceris lilii Scopoli (Coleoptera: Chrysomelidae), in North America" Insects 11, no. 9: 560. https://doi.org/10.3390/insects11090560