Tribological Properties of ZnS(NH2CH2CH2NH2)0.5 and ZnS as Additives in Lithium Grease
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemicals
2.2. Synthesis of ZnS(NH2CH2CH2NH2)0.5, ZnS, and the Grease Samples
2.3. Material Characterization and Tribological Tests
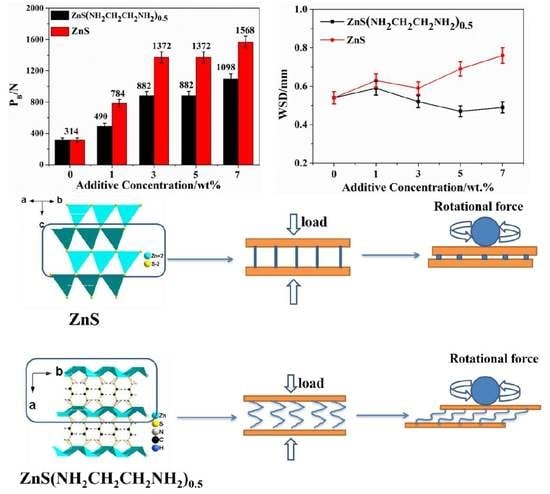
3. Results and Discussion
3.1. Characterizations of ZnS(NH2CH2CH2NH2)0.5 and ZnS
3.2. Tribological Properties of ZnS(NH2CH2CH2NH2)0.5 and ZnS
3.3. XPS Analyses of Worn Surface and Tribofilm
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jost, H. Tribology—Origin and future. Wear 1990, 136, 1–17. [Google Scholar] [CrossRef]
- Tomala, A.; Ripoll, M.R.; Gabler, C.; Remškar, M.; Kalin, M. Interactions between MoS2 nanotubes and conventional additives in model oils. Tribol. Int. 2017, 110, 140–150. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, C. The synthesis of MoS2, particles with different morphologies for tribological applications. Tribol. Int. 2017, 116, 285–294. [Google Scholar] [CrossRef]
- Hu, E.Z.; Xu, Y.; Hu, K.H.; Hu, X.G. Tribological properties of 3 types of MoS2 additives in different base greases. Lubr. Sci. 2017, 29, 1–15. [Google Scholar] [CrossRef]
- Quan, X.; Zhang, S.; Hu, M.; Gao, X.; Jiang, D.; Sun, J. Tribological properties of WS2/MoS2-Ag composite films lubricated with ionic liquids under vacuum conditions. Tribol. Int. 2017, 115, 389–396. [Google Scholar] [CrossRef]
- Aldana, P.U.; Vacher, B.; Mogne, T.L.; Belin, M.; Thiebaut, B.; Dassenoy, F. Action Mechanism of WS2 Nanoparticles with ZDDP Additive in Boundary Lubrication Regime. Tribol. Lett. 2014, 56, 49–58. [Google Scholar] [CrossRef]
- Zhou, L.H.; Wei, X.C.; Ma, Z.J.; Mei, B. Anti-friction performance of FeS nanoparticle synthesized by biological method. Appl. Surf. Sci. 2017, 407, 21–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, B.; Li, P.; Wang, X.; Zhang, Y. Tribological performance of CuS–ZnO nanocomposite film: The effect of CuS doping. Tribol. Int. 2013, 58, 7–11. [Google Scholar] [CrossRef]
- Liu, W.M.; Chen, S. An investigation of the tribological behaviour of surface-modified ZnS nanoparticles in liquid paraffin. Wear 2000, 238, 120–124. [Google Scholar] [CrossRef]
- Chen, S.; Liu, W. Characterization and antiwear ability of non-coated ZnS nanoparticles and DDP-coated ZnS nanoparticles. Mater. Res. Bull. 2001, 36, 137–143. [Google Scholar] [CrossRef]
- Min, Y.; Akbulut, M.; Prud’homme, R.K.; Golan, Y.; Israelachvili, J. Frictional properties of surfactant-coated rod-shaped nanoparticles in dry and humid dodecane. J. Phys. Chem. B 2008, 112, 14395–14401. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.B.; Gao, Y.P.; Li, Z.Y.; Zhou, A.G.; Li, P. Preparation and tribological properties of surface-modified ZnS nanoparticles. Lubr. Sci. 2015, 27, 241–250. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Li, G.T.; Zhang, G.; Wang, T.M.; Wang, Q.H. Hybrid effect of ZnS sub-micrometer particles and reinforcing fibers on tribological performance of polyimide under oil lubrication conditions. Wear 2017, 380–381, 86–95. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Z.; Ye, L.; Friedrich, K. Tribological properties of high temperature resistant polymer composites with fine particles. Tribol. Int. 2007, 40, 1170–1178. [Google Scholar] [CrossRef]
- Knör, N.; Gebhard, A.; Haupert, F.; Schlarb, A.K. Polyetheretherketone (PEEK) nanocomposites for extreme mechanical and tribological loads. Mech. Compos. Mater. 2009, 45, 199–206. [Google Scholar] [CrossRef]
- Yang, G.; Ma, H.; Wu, Z.; Zhang, P. Tribological behavior of ZnS-filled polyelectrolyte multilayers. Wear 2007, 262, 471–476. [Google Scholar] [CrossRef]
- Guo, Y.B.; Wang, D.G.; Liu, S.H.; Zhang, S.W. Synthesis and tribological properties of CuS/ZnS nanoparticles doped polyelectrolyte multilayers. Surf. Eng. 2013, 29, 17–22. [Google Scholar] [CrossRef]
- Kang, J.J.; Wang, C.B.; Wang, H.D.; Xu, B.S.; Liu, J.J.; Li, G.L. Research on tribological behaviors of composite Zn/ZnS coating under dry condition. Int. Vac. Congr. 2010, 285, 1940–1943. [Google Scholar] [CrossRef]
- Ouyang, X.; Tsai, T.Y.; Chen, D.H.; Huang, Q.J.; Cheng, W.H.; Clearfield, A. Ab initio structure study from in-house powder diffraction of a novel ZnS(EN)0.5 structure with layered wurtzite ZnS fragment. Chem. Commun. 2003, 7, 886–887. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, X.; Yu, J. A low-temperature and mild solvothermal route to the synthesis of wurtzite-type ZnS with single-crystalline nanoplate-like morphology. Cryst. Growth Des. 2005, 5, 1761–1765. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Xu, H.; Dong, J. Tribological Investigation of Two Different Layered Zirconium Phosphates as Grease Additives under Reciprocating Sliding Test. Tribol. Lett. 2016, 64. [Google Scholar] [CrossRef]
- Rudnick, L.R. Lubricant Additives: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Zhao, H.; Huang, X.; Wang, J.; Li, Y.; Liao, R.; Wang, X.; Qiu, G. Comparison of bioleaching and dissolution process of p-type and n-type chalcopyrite. Miner. Eng. 2017, 109, 153–161. [Google Scholar] [CrossRef]
- Wen, Z.; Xia, Y.; Liu, Z. Tribological Behavior and Mechanism of Overbased Complex Calcium Sulfonate Grease. Acta Pet. Sin. 2013, 29, 145–150. [Google Scholar]
- Gong, K.; Wu, X.; Zhao, G.; Wang, X. Tribological properties of polymeric aryl phosphates grafted onto multi-walled carbon nanotubes as high-performances lubricant additive. Tribol. Int. 2017, 116, 172–179. [Google Scholar] [CrossRef]
- Chen, X.J.; Xu, H.F.; Xu, N.S.; Zhao, F.H.; Lin, W.J.; Lin, G.; Fu, Y.L.; Huang, Z.L.; Wang, K.Z.; Wu, M.M. Wurtzite ZnS nanosaws produced by polar surfaces. Chem. Phys. Lett. 2004, 385, 8–11. [Google Scholar]
- Chen, X.; Xu, H.; Xu, N.; Zhao, F.; Lin, W.; Lin, G.; Wu, M. Kinetically Controlled Synthesis of Wurtzite ZnS Nanorods through Mild Thermolysis of a Covalent Organic−Inorganic Network. Inorg. Chem. 2003, 42, 3100–3106. [Google Scholar] [CrossRef]










| Element | ZnS a | ZnS b |
|---|---|---|
| XPS | XPS | |
| S | 2.07 | 9.30 |
| O | 81.95 | 72.63 |
| Fe | 13.43 | 9.63 |
| Zn | 2.54 | 8.44 |
| Element | ZnS(NH2CH2CH2NH2)0.5 a | ZnS(NH2CH2CH2NH2)0.5 b |
|---|---|---|
| XPS | XPS | |
| S | 2.36 | 3.81 |
| O | 82.92 | 82.51 |
| Fe | 12.29 | 9.98 |
| Zn | 2.43 | 3.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, A.; Niu, W.; Dai, Y.; Xu, H.; Dong, J. Tribological Properties of ZnS(NH2CH2CH2NH2)0.5 and ZnS as Additives in Lithium Grease. Lubricants 2019, 7, 26. https://doi.org/10.3390/lubricants7030026
Lu A, Niu W, Dai Y, Xu H, Dong J. Tribological Properties of ZnS(NH2CH2CH2NH2)0.5 and ZnS as Additives in Lithium Grease. Lubricants. 2019; 7(3):26. https://doi.org/10.3390/lubricants7030026
Chicago/Turabian StyleLu, Aoxiang, Wenxing Niu, Yingjing Dai, Hong Xu, and Jinxiang Dong. 2019. "Tribological Properties of ZnS(NH2CH2CH2NH2)0.5 and ZnS as Additives in Lithium Grease" Lubricants 7, no. 3: 26. https://doi.org/10.3390/lubricants7030026