Ecofriendly Protic Ionic Liquid Lubricants for Ti6Al4V
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Rheology
2.3. Contact Angle Measurements
2.4. Tribological Tests
2.5. Surface Analysis
3. Results and Discussion
3.1. Viscosity Measurements
3.2. Contact Angles
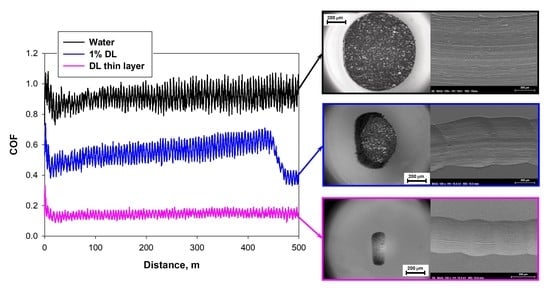
3.3. Friction Coefficients and Wear Rates at Room Temperature
3.3.1. Neat PIL Lubricants
3.3.2. Water + 1 wt.% PIL
3.3.3. Thin Lubricant Layers
3.4. Wear Mechanisms and Surface Analysis at Room Temperature
3.4.1. Neat PIL Lubricants
3.4.2. Water + 1 wt.% PILs
3.4.3. Thin-Layer Lubricants
3.5. Friction Coefficients and Wear Rates at 100 °C
3.6. Wear Mechanisms and Surface Analysis after Tests at 100 °C
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Long, M.; Rack, H.J. Friction and surface behaviour of selected titanium alloys during reciprocating-sliding motion. Wear 2001, 249, 157–167. [Google Scholar] [CrossRef]
- Chen, K.M.; Zhou, Y.; Li, X.X.; Zhang, Q.Y.; Wang, L.; Wang, S.Q. Investigation on wear characteristics of a titanium alloy/steel tribo-pair. Mater. Des. 2015, 65, 65–73. [Google Scholar] [CrossRef]
- Molinari, A.; Straffelini, G.; Tesi, B.; Bacci, T. Dry sliding wear mechanisms of the Ti6Al4V alloy. Wear 1997, 208, 105–112. [Google Scholar] [CrossRef]
- Raj, J.A.; Satish, V.K. Evolution of wear debris morphology during dry sliding of Ti-6Al-4V against SS316L under ambient and vacuum conditions. Wear 2020, 456–457, 203378. [Google Scholar]
- Yang, Y.; Zhang, C.H.; Dai, Y.J.; Luo, J.B. Lubricity and adsorption of castor oil sulfated sodium salt emulsion solution on titanium alloy. Tribol. Lett. 2019, 67, 61. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T.T.; Dai, Y.J.; Wang, Y.; Zhang, C.H. Effect of amines on the lubricity of castor oil-sulfated sodium salt solution for titanium alloys. Tribol. Lett. 2020, 68, 19. [Google Scholar] [CrossRef]
- Wickramasinghe, K.C.; Sasahara, H.; Abd Rahim, E.; Perera, G.I.P. Recent advances on high performance machining of aerospace materials and composites using vegetable oil-based metal working fluids. J. Clean. Prod. 2021, 310, 127459. [Google Scholar] [CrossRef]
- Singh, H.; Sharma, V.S.; Dogra, M. Exploration of graphene assisted vegetable oil based minimum quantity lubrication for surface grinding of Ti-6Al-4V-ELI. Tribol. Int. 2020, 144, 106113. [Google Scholar] [CrossRef]
- Mo, Y.; Tao, D. Tribological performance of nanotin as lubrication additives used in steel-copper tribo-pair. Ind. Lubr. Tribol. 2011, 63, 72–77. [Google Scholar] [CrossRef]
- Wrablewski, P. Investigation of energy losses of the internal combustion engine taking into account the correlation of the hydrophobic and hydrophilic. Energy 2023, 264, 126002. [Google Scholar] [CrossRef]
- Ibrahim, A.M.M.; Li, W.; Xiao, H.; Zeng, Z.; Ren, Y.; Alsoufi, M.S. Energy conservation and environmental sustainability during grinding operation of Ti-6Al-4V alloys vis eco-friendly oil/graphene nanoadditive and minimum quantity lubrication. Tribol. Int. 2020, 150, 106308. [Google Scholar] [CrossRef]
- Li, C.; Xu, J.; Chen, M.; An, Q.; El Mansori, M.; Ren, F. Tool wear processes in low frequency vibration assisted drilling of CFRP/Ti6Al4V stacks with forced air-cooling. Wear 2019, 426–427, 1616–1623. [Google Scholar] [CrossRef]
- Li, G.; Yi, S.; Li, N.; Pan, W.; Wen, C.; Ding, S. Quantitative analysis of cooling and lubrication effects of graphene oxide nano fluids in machining titanium alloy Ti6Al4V. J. Mater. Process. Technol. 2019, 271, 584–598. [Google Scholar] [CrossRef]
- Li, M.; Yu, T.; Yang, L.; Li, H.; Zhang, R.; Wang, W. Parameter optimization during minimum quantity lubrication milling of TC4 alloy with graphene-dispersed vegetable oil-based cutting fluid. J. Clean. Prod. 2019, 209, 1508–1522. [Google Scholar] [CrossRef]
- Sakib, S.; Ibne, Z.; Nurul, A.K.M.; Bashar, M.S.; Kabir, F.; Kamruzzaman, M. Tuning nano fluids for improved lubrication performance in turning biomedical grade titanium alloy. J. Clean. Prod. 2019, 206, 180–196. [Google Scholar]
- Quan, X.; Xie, H.M.; Xu, X.J.; Tang, J.Z. Study on the enhanced tribological performance for titanium alloys by PEG oil/Zn-nanoparticles. Mater. Res. Express 2020, 7, 126502. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Evolving structure-property relationships and expanding applications. Chem. Rev. 2015, 115, 11379–11448. [Google Scholar] [CrossRef]
- Cai, M.R.; Yu, Q.L.; Liu, W.M. Ionic liquid lubricants: When chemistry meets tribology. Chem. Soc. Rev. 2020, 49, 7753–7818. [Google Scholar] [CrossRef]
- Greer, A.J.; Jacquemin, J.; Hardacre, C. Industrial applications of ionic liquids. Molecules 2020, 25, 5207. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D. Ionic liquids as lubricants of titanium-steel contacts. Part 2. Friction, wear and surface interactions at high temperature. Tribol. Lett. 2010, 33, 111–126. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D. Ionic liquids as lubricants of titanium-steel contact. Part 3. Ti6Al4V lubricated with imidazolium ionic liquids with different alkyl chain lengths. Tribol. Lett. 2010, 40, 237–246. [Google Scholar] [CrossRef]
- Fan, M.; Jin, Y.; Han, Y.; Ma, L.; Li, W.; Lu, Y.; Zhou, F.; Liu, W. The effect of chemical structure on the tribological performance of perfluoropolysulfonate ILs as lubricants for Ti-6Al-4V tribopairs. J. Mol. Liq. 2021, 321, 114286. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Lu, Z.L.; Li, F.Z.; Jia, I.; Yang, Z.Q.; Chen, G.Q.; Yu, Q.L.; Dong, R.; Cai, M.R. Corrosion and lubrication properties of a halogen-free Gemini room-temeprature ionic liquid for titanium alloys. Tribol. Int. 2021, 156, 106850. [Google Scholar] [CrossRef]
- Davis, B.; Schueller, J.K.; Huang, Y. Study of ionic liquid as effective additive for minimum quantity lubrication during titanium machining. Manuf. Lett. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Gindri, I.M.; Siddiqui, D.A.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Improvement of tribological and anticorrosive performance of titanium surfaces coated with dicationic imidazolium-based ionic liquids. RSC Adv. 2016, 6, 78795–78802. [Google Scholar] [CrossRef]
- Nevshupa, M.; Conte, M.; del Campo, A.; Román, E. Analysis of tribochemical decomposition of two imidazolium ionic liquids on Ti-6Al-4V through mechanically stimulated gas emission spectrometry. Tribol. Int. 2016, 102, 19–27. [Google Scholar] [CrossRef]
- Duan, H.T.; Li, W.M.; Kumara, C.; Jin, Y.L.; Meyer, H.M.; Luo, H.M.; Qu, J. Ionic liquids as oil additives for lubricating oxygen-diffusion case-hardening titanium. Tribol. Int. 2019, 136, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, C.H.; Dai, Y.J.; Luo, J.B. Tribological properties of titanium alloys under lubrication of SEE oil and aqueous solutions. Tribol. Int. 2017, 109, 40–47. [Google Scholar] [CrossRef]
- Nor Hamran, N.N.; Ghani, J.A.; Ramli, R.; Haron, C.H.C. A review on recent development of minimum quantity lubrication for sustainable machining. J. Clean. Prod. 2020, 268, 122165. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Bai, Y.; Wu, Y.; Ma, Z.; Zhang, J.; Zhang, C.; Yu, B.; Zhou, F.; Liu, W.; et al. Towards superior lubricity and anticorrosion performances of proton-type ionic liquids additives for water-based lubricating fluids. Chem. Eng. J. 2020, 383, 123201. [Google Scholar] [CrossRef]
- Pavlovica, S.; Zicmanis, A.; Gzibovska, E.; Klavins, M.; Mekss, P. (2-Hydroxyethylammonium) lactates. Highly biodegradable and essentially non-toxic liquids. Green Sustain. Chem. 2011, 1, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Varela, B.; Brasil, G.; Andrade, R.S.; Barretto, R.M.; Trovatti, E.; Galdorfini, B.; Iglesias, M. Cytotoxic effect of protic ionic liquids in HepG2 and HaCat human cells: In vitro and in silico studies. Toxicol. Res. 2019, 8, 447–458. [Google Scholar]
- Guo, H.; Iglesias, P. Tribological behavior of ammonium-based protic ionic liquid as lubricant additive. Friction 2021, 9, 169–178. [Google Scholar] [CrossRef]
- Patel, A.; Guo, H.; Iglesias, P. Study of the lubricating ability of protic ionic liquid on an aluminum-steel contact. Lubricants 2018, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Li, S.S.; Chen, H.; Luo, T.; Wang, F.; Xiao, G.; Chen, Z.; Yi, M.; Sheng, C.; Xu, C. Tribological properties of 1-octyl-3-methylimidazolium lactate ionic liquid as a lubricant additive. J. Mol. Liq. 2021, 332, 115828. [Google Scholar] [CrossRef]
- Kaneko, M.; Akamatsu, M.; Sakai, K.; Sakai, H. Adsorption of hydrophilic amine-based protic ionic liquids on iron-based substrates. J. Oleo Sci. 2021, 70, 333–339. [Google Scholar] [CrossRef]
- Kaneko, K.; Sakai, K.; Sakai, H. Lubrication by adsorption films of hydrophilic amine-based protic ionic liquids: Effect of anion species. J. Oleo Sci. 2021, 70, 1615–1621. [Google Scholar] [CrossRef]
- Iglesias, M.; Gonzalez-Olmos, R.; Cota, I.; Medina, F. Bronsted ionic liquids: Study of physico-chemical properties and catalytic activity in aldol condensations. Chem. Eng. J. 2010, 162, 802–808. [Google Scholar] [CrossRef]
- Álvarez, V.H.; Dosil, N.; Gonzalez-Cabaleiro, R.; Mattedi, S.; Martin-Pastor, M.; Iglesias, M.; Navaza, J.M. Bronsted ionic liquids for sustainable processes: Synthesis and physical properties. J. Chem. Eng. Data 2010, 55, 625–632. [Google Scholar] [CrossRef]
- Andrade, R.; Torres, D.; Ribeiro, F.R.; Chiari-Andreo, B.G.; Oshiro, J.A.; Iglesias, M. Sustainable cotton dyeing in nonaqueous medium applying protic ionic liquids. ACS Sustain. Chem. Eng. 2017, 5, 8756–8765. [Google Scholar] [CrossRef]
- Camargo, D.; Andrade, R.S.; Ferreira, G.A.; Mazzer, H.; Cardozo-Filho, L.; Iglesias, M. Investigation of the rheological properties of protic ionic liquids. J. Phys. Org. Chem. 2016, 29, 604–612. [Google Scholar] [CrossRef]
- Martínez-Rubio, P.M.; Avilés, M.D.; Arias-Pardilla, J.; Carrión-Vilches, F.J.; Sanes, J.; Bermúdez, M.D.; Pamies, R. Physicochemical characterisation of graphene-ammonium lactate ionic liquid nanofluid. J. Mol. Liq. 2022, 367, 120446. [Google Scholar] [CrossRef]
- Espinosa, T.; Jiménez, A.E.; Sanes, J.; Jiménez, A.E.; Iglesias, M.; Bermúdez, M.D. Ultralow friction with a protic ionic liquid boundary film at the water-lubricated sapphire-stainless steel interface. Tribol. Lett. 2014, 53, 1–9. [Google Scholar] [CrossRef]
- Saurín, N.; Avilés, M.D.; Espinosa, T.; Sanes, J.; Carrión, F.J.; Bermúdez, M.D.; Iglesias, P. Carbon nanophases in ordered nanofluid lubricants. Wear 2017, 376–377, 747–755. [Google Scholar] [CrossRef]
- Wagner, C.D.; Naumkin, A.V.; Kraut-Vass, A.; Allison, J.W.; Powell, C.J.; Rumble, J.R., Jr. NIST Standard Reference Database (web version) 2003. Available online: http:/srdata.nist.gov/xps/ (accessed on 28 November 2022).


















| Ionic Liquid | A (Pa·s) | n |
|---|---|---|
| DCi | 380 ± 40 | 0.74 ± 0.02 |
| DL | 11.8 ± 0.6 | 0.89 ± 0.01 |
| DSa | 9.6 ± 0.4 | 0.845 ± 0.008 |
| Ionic Liquid | Viscosity (Pa·s) |
|---|---|
| DCi | 1.42 ± 0.05 |
| DL | 0.067 ± 0.004 |
| DSa | 0.051 ± 0.003 |
| Lubricant | Initial | After 5 min |
|---|---|---|
| DSa |  61.3 (±3.4) |  16.3 (±1.6) |
| DCi |  95.2 (±5.6) |  73.1 (±1.4) |
| DL |  78.8 (±0.3) |  40.0 (±0.5) |
| Water + 1%DSa |  38.2 (±1.4) |  30.9 (±1.8) |
| Water + 1%DCi |  32.7 (±0.7) |  23.9 (±1.7) |
| Water + 1%DL |  33.9 (±1.4) |  28.5 (±1.2) |
| Lubricant | Coefficient of Friction (Standard Deviation) | Wear Rate (mm3/m) (Standard Deviation) |
|---|---|---|
| Water | 0.90 (0.04) | 5.2 × 10−4 (1.8 × 10−5) |
| DSa | 0.28 (0.07) | 6.1 × 10−4 (2.9 × 10−5) |
| DCi | 0.28 (0.03) | 3.7 × 10−4 (4.7 × 10−6) |
| DL | 0.30 (0.01) | 2.9 × 10−4 (3.6 × 10−5) |
| Lubricant | Coefficient of Friction (Standard Deviation) | Wear Rate (mm3/m) (Standard Deviation) |
|---|---|---|
| Water + DSa | 0.43 (0.03) | 3.6 × 10−4 (2.3 × 10−5) |
| Water + DCi | 0.65 (0.06) | 5.8 × 10−4 (5.2 × 10−5) |
| Water + DL | 0.54 (0.05) | 6.4 × 10−4 (1.7 × 10−5) |
| Lubricant | Coefficient of Friction (Standard Deviation) | Wear Rate (mm3/m) (Standard Deviation) |
|---|---|---|
| DSa thin layer | 0.42 (0.02) | 3.2 × 10−4 (1.8 × 10−5) |
| DCi thin layer | 0.24 (0.04) | 2.2 × 10−4 (6.6 × 10−6) |
| DL thin layer | 0.13 (0.01) | 2.4 × 10−4 (3.3 × 10−5) |
| Element | Neat DL Lubricant | DL Thin Layer Lubricant | ||||||
|---|---|---|---|---|---|---|---|---|
| Outside the Wear Track | Inside the Wear Track | Outside the Wear Track | Inside the Wear Track | |||||
| Binding Energy (eV) | Atomic % | Binding Energy (eV) | Atomic % | Binding Energy (eV) | Atomic % | Binding Energy (eV) | Atomic % | |
| C1s | 285.0 | 29.4 | 285.0 | 28.6 | 285 | 19.6 | 285.0 | 23.6 |
| 286.4 | 10.9 | 286.4 | 10.9 | 286.5 | 8.8 | 286.5 | 8.0 | |
| 288.0 | 0.9 | 287.8 | 0.5 | 287.8 | 0.7 | 288.0 | 1.0 | |
| 288.9 | 2.1 | 288.8 | 2.1 | 288.6 | 2.4 | 288.7 | 2.1 | |
| N1s | 400.1 | 1.1 | 400.1 | 1.0 | 400.1 | 1.0 | 400.1 | 1.2 |
| 401.5 | 0.3 | 401.5 | 0.2 | 401.8 | 0.3 | 401.8 | 0.3 | |
| Al2p3/2 | 71.7 | 0.7 | 71.5 | 0.8 | 71.6 | 0.7 | 71.7 | 0.6 |
| 74.0 | 2.4 | 74.0 | 2.8 | 74,06 | 3.1 | 74.2 | 4.1 | |
| 76.5 | 0.5 | |||||||
| Ti2p3/2 | 453.5 | 0.7 | 453.5 | 0.7 | 453.6 | 0.7 | 453.7 | 0.7 |
| 458.7 | 9.6 | 458.7 | 9.3 | 458.8 | 13.2 | 458.8 | 12.7 | |
| O1s | 530.3 | 26.0 | 530.2 | 25.2 | 530.3 | 35.2 | 530.4 | 31.7 |
| 531.3 | 3.3 | 531.2 | 4.4 | 531.6 | 6.9 | 531.5 | 4.8 | |
| 532.6 | 12.5 | 532.5 | 13.3 | 532.9 | 7.3 | 532.6 | 8.6 | |
| Lubricant | Coefficient of Friction (Standard Deviation) | Wear Rate (mm3/m) (Standard Deviation) |
|---|---|---|
| DSa | 0.52 (0.02) | 5.7 × 10−4 (8.6 × 10−5) |
| DCi | 0.36 (0.02) | 4.2 × 10−4 (2.9 × 10−5) |
| DL | 0.43 (0.03) | 4.0 × 10−4 (4.1 × 10−5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, A.-E.; Avilés, M.-D.; Pamies, R.; Bermúdez, M.-D.; Carrión-Vilches, F.-J.; Sanes, J. Ecofriendly Protic Ionic Liquid Lubricants for Ti6Al4V. Lubricants 2023, 11, 5. https://doi.org/10.3390/lubricants11010005
Jiménez A-E, Avilés M-D, Pamies R, Bermúdez M-D, Carrión-Vilches F-J, Sanes J. Ecofriendly Protic Ionic Liquid Lubricants for Ti6Al4V. Lubricants. 2023; 11(1):5. https://doi.org/10.3390/lubricants11010005
Chicago/Turabian StyleJiménez, Ana-Eva, María-Dolores Avilés, Ramón Pamies, María-Dolores Bermúdez, Francisco-José Carrión-Vilches, and José Sanes. 2023. "Ecofriendly Protic Ionic Liquid Lubricants for Ti6Al4V" Lubricants 11, no. 1: 5. https://doi.org/10.3390/lubricants11010005