Acquisition Parameters Influence Diffusion Metrics Effectiveness in Probing Prostate Tumor and Age-Related Microstructure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Recruitment
2.2. MRI Protocol
2.3. Image Analysis and DTI-Metrics Quantification
2.4. MR-Targeted Biopsy and Classification of PCa Lesions
2.5. Signal-to-Noise Ratio, Contrast Ratio, and Coefficient of Variation
2.6. Statistical Analysis
3. Results
3.1. Visual Quality of DWIs and DTI Parametric Maps of Prostate with Lesions
3.2. Discrimination of PCa Lesions Versus Non-Cancerous Tissue
3.3. Discrimination between Different Degrees of Malignancy
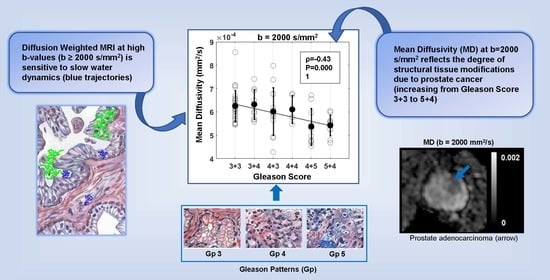
3.4. Correlation between DTI Parameters and Gleason Score (GS)
3.5. Association between the DTI Parameters and Patients’ Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Diffusion Model
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef]
- Borghesi, M.; Ahmed, H.; Nam, R.; Schaeffer, E.; Schiavina, R.; Taneja, S.; Weidner, W.; Loeb, S. Complications after systematic, random, and image-guided prostate biopsy. Eur. Urol. 2017, 71, 353–365. [Google Scholar] [CrossRef]
- Loeb, S.; Vellekoop, A.; Ahmed, H.U.; Catto, J.; Emberton, M.; Nam, R.; Rosario, D.J.; Scattoni, V.; Lotan, Y. Systematic review of complications of prostate biopsy. Eur. Urol. 2013, 64, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D. EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S. EAU–ESTRO–SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2016, 71, 618–629. [Google Scholar] [CrossRef]
- Boesen, L. Multiparametric MRI in detection and staging of prostate cancer. Dan. Med. Bull. 2017, 64, B5327. [Google Scholar]
- Caglic, I.; Kovac, V.; Barrett, T. Multiparametric MRI-local staging of prostate cancer and beyond. Radiol. Oncol. 2019, 53, 159–170. [Google Scholar] [CrossRef]
- Futterer, J.J.; Briganti, A.; De Visschere, P.; Emberton, M.; Giannarini, G.; Kirkham, A.; Taneja, S.S.; Thoeny, H.; Villeirs, G.; Villers, A. Can Clinically Significant Prostate Cancer Be Detected with Multiparametric Magnetic Resonance Imaging? A Systematic Review of the Literature. Eur. Urol. 2015, 68, 1045–1053. [Google Scholar] [CrossRef]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Steiger, P.; Thoeny, H.C. Prostate MRI based on PI-RADS Version 2: How we review and report. Cancer Imaging 2016, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Le Bihan, D.; Poupon, C.; Amadon, A.; Lethimonnier, F. Artifacts and pitfalls in diffusion MRI. J. Magn. Reson. Imaging 2006, 24, 478–488. [Google Scholar] [CrossRef]
- Katahira, K.; Takahara, T.; Kwee, T.C.; Oda, S.; Suzuki, Y.; Morishita, S.; Kitani, K.; Hamada, Y.; Kitaoka, M.; Yamashita, Y. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: Evaluation in 201 cases with histopathological correlation. Eur. Radiol. 2011, 21, 188–196. [Google Scholar] [CrossRef]
- Blackledge, M.D.; Leach, M.O.; Collins, D.J.; Koh, D.-M. Computed diffusion-weighted MR imaging may improve tumor detection. Radiology 2011, 261, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Parikh, N.; Kierans, A.S.; Kong, M.X.; Babb, J.S.; Taneja, S.S.; Ream, J.M. Prostate Cancer Detection Using Computed Very High b-value Diffusion-weighted Imaging: How High Should We Go? Acad. Radiol. 2016, 23, 704–711. [Google Scholar] [CrossRef]
- Vural, M.; Ertaş, G.; Onay, A.; Acar, Ö.; Esen, T.; Sağlıcan, Y.; Zengingönül, H.P.; Akpek, S. Conspicuity of peripheral zone prostate cancer on computed diffusion-weighted imaging: Comparison of cDWI1500, cDWI2000, and cDWI3000. BioMed Res. Int. 2014, 2014, 768291. [Google Scholar] [CrossRef] [PubMed]
- Gürses, B.; Tasdelen, N.; Yencilek, F.; Kılıckesmez, N.O.; Alp, T.; Fırat, Z.; Albayrak, M.S.; Uluğ, A.M.; Gürmen, A.N. Diagnostic utility of DTI in prostate cancer. Eur. J. Radiol. 2011, 79, 172–176. [Google Scholar] [CrossRef]
- Li, L.; Margolis, D.J.; Deng, M.; Cai, J.; Yuan, L.; Feng, Z.; Min, X.; Hu, Z.; Hu, D.; Liu, J. Correlation of gleason scores with magnetic resonance diffusion tensor imaging in peripheral zone prostate cancer. J. Magn. Reson. Imaging 2015, 42, 460–467. [Google Scholar] [CrossRef]
- Uribe, C.F.; Jones, E.C.; Chang, S.D.; Goldenberg, S.L.; Reinsberg, S.A.; Kozlowski, P. In vivo 3T and ex vivo 7T diffusion tensor imaging of prostate cancer: Correlation with histology. Magn. Reson. Imaging 2015, 33, 577–583. [Google Scholar] [CrossRef]
- Bajgiran, A.M.; Mirak, S.A.; Sung, K.; Sisk, A.E.; Reiter, R.E.; Raman, S.S. Apparent diffusion coefficient (ADC) ratio versus conventional ADC for detecting clinically significant prostate cancer with 3-T MRI. Am. J. Roentgenol. 2019, 213, W134–W142. [Google Scholar] [CrossRef]
- Nezzo, M.; Di Trani, M.; Caporale, A.; Miano, R.; Mauriello, A.; Bove, P.; Capuani, S.; Manenti, G. Mean diffusivity discriminates between prostate cancer with grade group 1&2 and grade groups equal to or greater than 3. Eur. J. Radiol. 2016, 85, 1794–1801. [Google Scholar]
- Jenkinson, M.; Beckmann, C.F.; Behrens, T.E.; Woolrich, M.W.; Smith, S.M. Fsl. Neuroimage 2012, 62, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, P.T. Principles of Nuclear Magnetic Resonance Microscopy; Oxford University Press on Demand: Oxford, UK, 1993; pp. 158–334. [Google Scholar]
- Capuani, S.; Manenti, G.; Iundusi, R.; Tarantino, U. Focus on diffusion MR investigations of musculoskeletal tissue to improve osteoporosis diagnosis: A brief practical review. BioMed Res. Int. 2015, 2015, 948610. [Google Scholar] [CrossRef] [PubMed]
- Hammerich, K.H.; Ayala, G.E.; Wheeler, T.M. Anatomy of the prostate gland and surgical pathology of prostate cancer. In Prostate Cancer; Hricak, H., Scardino, P.T., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 1–10. [Google Scholar]
- Hambrock, T.; Somford, D.M.; Huisman, H.J.; van Oort, I.M.; Witjes, J.A.; Hulsbergen-van de Kaa, C.A.; Scheenen, T.; Barentsz, J.O. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 2011, 259, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, Y.; Vargas, H.A.; Nyman, G.; Akin, O.; Hricak, H. Image artifacts on prostate diffusion-weighted magnetic resonance imaging: Trade-offs at 1.5 Tesla and 3.0 Tesla. Acad. Radiol. 2013, 20, 1041–1047. [Google Scholar] [CrossRef]
- Metens, T.; Miranda, D.; Absil, J.; Matos, C. What is the optimal b value in diffusion-weighted MR imaging to depict prostate cancer at 3T? Eur. Radiol. 2012, 22, 703–709. [Google Scholar] [CrossRef]
- Hasan, K.M.; Alexander, A.L.; Narayana, P.A. Does fractional anisotropy have better noise immunity characteristics than relative anisotropy in diffusion tensor MRI? An analytical approach. Magn. Reson. Med. 2004, 51, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.K.; Knösche, T.R.; Turner, R. White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage 2013, 73, 239–254. [Google Scholar] [CrossRef]
- Jones, D.K.; Basser, P.J. “Squashing peanuts and smashing pumpkins”: How noise distorts diffusion-weighted MR data. Magn. Reson. Med. 2004, 52, 979–993. [Google Scholar] [CrossRef]
- Reischauer, C.; Wilm, B.J.; Froehlich, J.M.; Gutzeit, A.; Prikler, L.; Gablinger, R.; Boesiger, P.; Wentz, K.-U. High-resolution diffusion tensor imaging of prostate cancer using a reduced FOV technique. Eur. J. Radiol. 2011, 80, e34–e41. [Google Scholar] [CrossRef]
- Descoteaux, M.; Deriche, R.; Knosche, T.R.; Anwander, A. Deterministic and probabilistic tractography based on complex fibre orientation distributions. IEEE Trans. Med. Imaging 2009, 28, 269–286. [Google Scholar] [CrossRef]
- Sklinda, K.; Frączek, M.; Mruk, B.; Walecki, J. Normal 3T MR anatomy of the prostate gland and surrounding structures. Adv. Med. 2019, 2019, 3040859. [Google Scholar] [CrossRef]
- Kitajima, K.; Kaji, Y.; Kuroda, K.; Sugimura, K. High b-value diffusion-weighted imaging in normal and malignant peripheral zone tissue of the prostate: Effect of signal-to-noise ratio. Magn. Reson. Med. Sci. 2008, 7, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.R.; Tamada, T.; Takahashi, S.; Tanaka, U.; Sofue, K.; Kanda, T.; Nogami, M.; Ohno, Y.; Hinata, N.; Fujisawa, M. Computed diffusion-weighted imaging in prostate cancer: Basics, advantages, cautions, and future prospects. Korean J. Radiol. 2018, 19, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Iima, M.; Yano, K.; Kataoka, M.; Umehana, M.; Murata, K.; Kanao, S.; Togashi, K.; Le Bihan, D. Quantitative non-Gaussian diffusion and intravoxel incoherent motion magnetic resonance imaging: Differentiation of malignant and benign breast lesions. Investig. Radiol. 2015, 50, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Rosenkrantz, A.B.; Sigmund, E.E.; Johnson, G.; Babb, J.S.; Mussi, T.C.; Melamed, J.; Taneja, S.S.; Lee, V.S.; Jensen, J.H. Prostate cancer: Feasibility and preliminary experience of a diffusional kurtosis model for detection and assessment of aggressiveness of peripheral zone cancer. Radiology 2012, 264, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Di Trani, M.G.; Nezzo, M.; Caporale, A.S.; De Feo, R.; Miano, R.; Mauriello, A.; Bove, P.; Manenti, G.; Capuani, S. Performance of diffusion kurtosis imaging versus diffusion tensor imaging in discriminating between benign tissue, low and high Gleason grade prostate cancer. Acad. Radiol. 2019, 26, 1328–1337. [Google Scholar] [CrossRef]
- Le Bihan, D.; Iima, M. Diffusion magnetic resonance imaging: What water tells us about biological tissues. PLoS Biol 2015, 13, e1002203. [Google Scholar]
- Bourne, R. Magnetic resonance microscopy of prostate tissue: How basic science can inform clinical imaging development. J. Med. Radiat. Sci. 2013, 60, 5–10. [Google Scholar] [CrossRef]
- Chatterjee, A.; Watson, G.; Myint, E.; Sved, P.; McEntee, M.; Bourne, R. Changes in epithelium, stroma, and lumen space correlate more strongly with gleason pattern and are stronger predictors of prostate ADC changes than cellularity metrics. Radiology 2015, 277, 751–762. [Google Scholar] [CrossRef]
- Bourne, R.M.; Kurniawan, N.; Cowin, G.; Stait-Gardner, T.; Sved, P.; Watson, G.; Price, W.S. Microscopic diffusivity compartmentation in formalin-fixed prostate tissue. Magn. Reson. Med. 2012, 68, 614–620. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Konety, B.R.; Crook, J.; Saad, F.; Lawrentschuk, N. Landmarks in prostate cancer. Nat. Rev. Urol. 2018, 15, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Rane, J.K.; Pellacani, D.; Maitland, N.J. Advanced prostate cancer—A case for adjuvant differentiation therapy. Nat. Rev. Urol. 2012, 9, 595–602. [Google Scholar] [CrossRef]
- Kim, C.; Jang, S.; Park, B. Diffusion tensor imaging of normal prostate at 3 T: Effect of number of diffusion-encoding directions on quantitation and image quality. Br. J. Radiol. 2014, 85, e279–e283. [Google Scholar] [CrossRef]
- Lemberskiy, G.; Rosenkrantz, A.B.; Veraart, J.; Taneja, S.S.; Novikov, D.S.; Fieremans, E. Time-dependent diffusion in prostate cancer. Investig. Radiol. 2017, 52, 405–411. [Google Scholar] [CrossRef]
- Bourne, R.; Liang, S.; Panagiotaki, E.; Bongers, A.; Sved, P.; Watson, G. Measurement and modeling of diffusion time dependence of apparent diffusion coefficient and fractional anisotropy in prostate tissue ex vivo. NMR Biomed. 2017, 30, e3751. [Google Scholar] [CrossRef] [PubMed]
- Shenhar, C.; Degani, H.; Ber, Y.; Baniel, J.; Tamir, S.; Benjaminov, O.; Rosen, P.; Furman-Haran, E.; Margel, D. Diffusion is directional: Innovative diffusion tensor imaging to improve prostate cancer detection. Diagnostics 2021, 11, 563. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W. A contemporary prostate cancer grading system: A validated alternative to the Gleason score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tian, W.-Z.; Hu, C.-H.; Niu, T.-L.; Wang, X.-L.; Chen, X.-Y. Age-related changes of normal prostate: Evaluation by MR diffusion tensor imaging. Int. J. Clin. Exp. Med. 2015, 8, 11220. [Google Scholar] [PubMed]
- Awedew, A.F.; Han, H.; Abbasi, B.; Abbasi-Kangevari, M.; Ahmed, M.B.; Almidani, O.; Amini, E.; Arabloo, J.; Argaw, A.M.; Athari, S.S. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2022, 3, e754–e776. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, B.; Forde, J.C.; Thomas, D.D.M.; Laor, L.; Hossack, T.; Woo, H.H.; Te, A.E.; Kaplan, S.A. Benign prostatic hyperplasia. Nat. Rev. Dis. Prim. 2016, 2, 1–15. [Google Scholar] [CrossRef]
- Hedgire, S.; Kilcoyne, A.; Tonyushkin, A.; Mao, Y.; Uyeda, J.W.; Gervais, D.A.; Harisinghani, M.G. Effect of androgen deprivation and radiation therapy on MRI fiber tractography in prostate cancer: Can we assess treatment response on imaging? Br. J. Radiol. 2019, 92, 20170170. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Greer, P.B.; Simpson, J.; Denham, J.; Lau, P.; Dowling, J.; Hondermarck, H.; Ramadan, S. Characterization of prostate cancer using diffusion tensor imaging: A new perspective. Eur. J. Radiol. 2019, 110, 112–120. [Google Scholar] [CrossRef]
- Jones, D. How many shells?—Investigating a long held tradition in DT-MRI. Proc. Intl. Soc. Mag. Reson. Med. 2007, 15, 4. [Google Scholar]
- Correia, M.M.; Carpenter, T.A.; Williams, G.B. Looking for the optimal DTI acquisition scheme given a maximum scan time: Are more b-values a waste of time? Magn. Reson. Imaging 2009, 27, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Tamada, T.; Sofue, K.; Murakami, T. Diffusion and quantification of diffusion of prostate cancer. Br. J. Radiol. 2022, 95, 20210653. [Google Scholar] [CrossRef] [PubMed]
- Caporale, A.; Bonomo, G.B.; Tani Raffaelli, G.; Tata, A.M.; Avallone, B.; Wehrli, F.W.; Capuani, S. Transient anomalous diffusion MRI in excised mouse spinal cord: Comparison among different diffusion metrics and validation with histology. Front. Neurosci. 2022, 15, 1869. [Google Scholar] [CrossRef]
- Palombo, M.; Barbetta, A.; Cametti, C.; Favero, G.; Capuani, S. Transient anomalous diffusion MRI measurement discriminates porous polymeric matrices characterized by different sub-microstructures and fractal dimension. Gels 2022, 8, 95. [Google Scholar] [CrossRef]
- Hectors, S.J.; Semaan, S.; Song, C.; Lewis, S.; Haines, G.K.; Tewari, A.; Rastinehad, A.R.; Taouli, B. Advanced diffusion-weighted imaging modeling for prostate cancer characterization: Correlation with quantitative histopathologic tumor tissue composition—A hypothesis-generating study. Radiology 2018, 286, 918–928. [Google Scholar] [CrossRef]
- Palombo, M.; Gabrielli, A.; De Santis, S.; Capuani, S. The γ parameter of the stretched-exponential model is influenced by internal gradients: Validation in phantoms. J. Magn. Reson. 2012, 216, 28–36. [Google Scholar] [CrossRef]
- Costantini, G.; Capuani, S.; Farrelly, F.; Taloni, A. Nuclear magnetic resonance signal decay in the presence of a background gradient: Normal and anomalous diffusion. J. Chem. Phys. 2023, 158, 174106. [Google Scholar] [CrossRef] [PubMed]
- Palombo, M.; Valindria, V.; Singh, S.; Chiou, E.; Giganti, F.; Pye, H.; Whitaker, H.C.; Atkinson, D.; Punwani, S.; Alexander, D.C.; et al. Joint estimation of relaxation and diffusion tissue parameters for prostate cancer with relaxation-VERDICT MRI. Sci. Rep. 2023, 13, 2999. [Google Scholar] [CrossRef] [PubMed]








| GS a | Three Levels Grade (L, I, H) b | Number of Patients | Number of PZ c Lesions | Number of CG d Lesions | Number of PZ + CG Slices |
|---|---|---|---|---|---|
| 3 + 3 | L | 16 | 10 (17 slices) | 6 (12 slices) | 29 |
| 3 + 4 | I | 5 | 8 (7 slices) | 5 (3 slices) | 10 |
| 4 + 3 | H | 4 | 8 (13 slices) | 0 | 13 |
| 4 + 4 | H | 3 | 5 (5 slices) | 0 | 5 |
| 4 + 5 | H | 2 | 2 (10 slices) | 0 | 10 |
| 5 + 4 | H | 2 | 1 (8 slices) | 1 (2 slices) | 10 |
| Tot. | 32 | 34 (60 slices) | 12 (17 slices) | 77 |
| b-Value (s/mm2) | CNRDWI a | CRDWI | CRMD | CRFA | CRMD b-Range b | CRFA b-Range b |
|---|---|---|---|---|---|---|
| 500 | 11.6 ± 2.0 | 0.13 ± 0.08 | 3.82 ± 0.18 | 1.03 ± 0.09 | - | - |
| 1000 | 15.4 ± 1.5 | 0.26 ± 0.12 | 4.48 ± 0.21 | 0.78 ± 0.07 | 4.32 ± 0.17 | 0.85 ± 0.08 |
| 1500 | 14.4 ± 2.0 | 0.33 ± 0.14 | 4.57 ± 0.17 | 0.68 ± 0.06 | 4.17 ± 0.17 | 0.79 ± 0.08 |
| 2000 | 12.7 ± 2.3 | 0.35 ± 0.15 | 4.44 ± 0.16 | 0.58 ± 0.07 | 3.79 ± 0.18 | 0.66 ± 0.07 |
| 2500 | 9.6 ± 1.6 | 0.34 ± 0.14 | 4.62 ± 0.20 | 0.74 ± 0.07 | 3.21 ± 0.18 | 0.60 ± 0.06 |
| b-Value (s/mm2) | M | D// | D┴ | FA | |
|---|---|---|---|---|---|
| 500 | GS | - | - | - | 3 + 4/5 + 4 (0.03) 4 + 5/5 + 4 (0.02) |
| LIH a | - | - | - | - | |
| 1000 | GS | - | - | - | - |
| LIH | - | - | L/H (0.04) | - | |
| 1500 | GS | - | 3 + 4/5 + 4 (0.009) | 3 + 4/5 + 4 (0.03) | 4 + 5/5 + 4 (0.02) |
| LIH | - | I/H (0.02) | L/H (0.04) I/H (0.02) | - | |
| 2000 | GS | 3 + 3/4 + 5 (0.02) 3 + 3/5 + 4 (0.04) | 3 + 3/5 + 4 (0.002) 3 + 4/4 + 5 (0.02) 3 + 4/5 + 4 (0.0007) 4 + 4/5 + 4 (0.03) | 3 + 3/4 + 5 (0.02) 3 + 3/5 + 4 (0.002) 3 + 4/4 + 5 (0.03) 3 + 4/5 + 4 (0.006) | 3 + 3/5 + 4 (0.02) |
| LIH | L/H (0.01) | L/H (0.01) I/H (0.003) | L/H (0.002) I/H (0.01) | - | |
| 2500 | GS | 3 + 3/5 + 4 (0.04) | 3 + 3/5 + 4 (0.004) 3 + 4/5 + 4 (0.01) | 3 + 3/5 + 4 (0.005) | 3 + 4/5 + 4 (0.02) |
| LIH | L/H (0.01) | L/H (0.009) I/H (0.03) | L/H (0.003) | - | |
| b-values range (s/mm2) | MD | D// | D┴ | FA | |
| 0–1000 | GS | - | - | - | 3 + 4/5 + 4 (0.007) 4 + 5/5 + 4 (0.045) |
| LIH a | - | L/H (0.04) | L/H (0.03) | I/H (0.02) | |
| 0–1500 | GS | - | 3 + 3/5 + 4 (0.007) 3 + 4/5 + 4 (0.003) | 3 + 3/5 + 4 (0.006) 3 + 4/5 + 4 (0.004) | - |
| LIH | L/H (0.03) I/H (0.03) | L/H (0.02) I/H (0.009) | L/H (0.003) I/H (0.003) | - | |
| 0–2000 | GS | 3 + 3/5 + 4 (0.02) 3 + 4/5 + 4 (0.045) | 3 + 3/5 + 4 (0.001) 3 + 4/5 + 4 (0.0006) | 3 + 3/5 + 4 (0.001) 3 + 4/5 + 4 (0.002) | 3 + 4/5 + 4 (0.049) |
| LIH | L/H (0.007) I/H (0.04) | L/H (0.02) I/H (0.006) | L/H (0.002) I/H (0.006) | - | |
| 0–2500 | GS | 3 + 3/5 + 4 (0.01) | 3 + 3/5 + 4 (0.0006) 3 + 4/5 + 4 (0.002) | 3 + 3/5 + 4 (0.001) 3 + 4/5 + 4 (0.02) | 3 + 4/5 + 4 (0.006) |
| LIH | L/H (0.008) | L/H (0.007) I/H (0.03) | L/H (0.002) | I/H (0.03) | |
| b-value (s/mm2) | MD | D// | D┴ | FA | ||||||||
| AUC | Sp | Se | AUC | Sp | Se | AUC | Sp | Se | AUC | Sp | Se | |
| 500 | 0.59 | 0.9 | 0.37 | 0.66 | 0.84 | 0.47 | 0.65 | 0.89 | 0.45 | 0.61 | 0.42 | 0.79 |
| 1000 | 0.64 | 0.9 | 0.4 | 0.69 | 0.89 | 0.53 | 0.69 | 0.87 | 0.47 | 0.67 | 0.79 | 0.53 |
| 1500 | 0.68 | 0.97 | 0.42 | 0.71 | 0.87 | 0.53 | 0.72 | 0.82 | 0.63 | 0.61 | 0.76 | 0.53 |
| 2000 | 0.72 | 0.66 | 0.74 | 0.74 | 1 | 0.47 | 0.75 | 0.76 | 0.71 | 0.67 | 0.74 | 0.63 |
| 2500 | 0.70 | 0.68 | 0.66 | 0.73 | 0.84 | 0.55 | 0.73 | 0.79 | 0.63 | 0.65 | 0.76 | 0.58 |
| b-value range (s/mm2) | MD | D// | D┴ | FA | ||||||||
| AUC | Sp | Se | AUC | Sp | Se | AUC | Sp | Se | AUC | Sp | Se | |
| 0–1000 | 0.68 | 0.68 | 0.66 | 0.72 | 0.95 | 0.5 | 0.73 | 0.92 | 0.47 | 0.68 | 0.58 | 0.76 |
| 0–1500 | 0.74 | 0.63 | 0.82 | 0.75 | 0.82 | 0.71 | 0.78 | 0.74 | 0.76 | 0.63 | 0.55 | 0.74 |
| 0–2000 | 0.76 | 0.71 | 0.76 | 0.74 | 0.92 | 0.58 | 0.77 | 0.84 | 0.68 | 0.62 | 0.66 | 0.63 |
| 0–2500 | 0.73 | 0.68 | 0.79 | 0.72 | 0.84 | 0.58 | 0.73 | 0.55 | 0.82 | 0.67 | 0.84 | 0.47 |
| b-Value (s/mm2) | MD | D// | D┴ | FA | ||||
| 𝜌 | P | 𝜌 | P | 𝜌 | P | 𝜌 | P | |
| 500 | −0.22 | 0.06 | −0.33 | 0.003 | −0.31 | 0.006 | −0.18 | 0.13 |
| 1000 | −0.29 | 0.01 | −0.35 | 0.002 | −0.36 | 0.002 | −0.25 | 0.03 |
| 1500 | −0.33 | 0.003 | −0.37 | 0.001 | −0.39 | 0.0004 | −0.18 | 0.1 |
| 2000 | −0.43 | 0.0001 | −0.45 | <0.0001 | −0.49 | <0.0001 | −0.29 | 0.01 |
| 2500 | −0.39 | 0.0006 | −0.40 | 0.0003 | −0.43 | 0.0001 | −0.23 | 0.04 |
| b-value range (s/mm2) | MD | D// | D┴ | FA | ||||
| 𝜌 | P | 𝜌 | P | 𝜌 | P | 𝜌 | P | |
| 0–1000 | −0.33 | 0.004 | −0.38 | 0.0007 | −0.40 | 0.0003 | −0.27 | 0.02 |
| 0–1500 | −0.42 | 0.0002 | −0.44 | <0.0001 | −0.49 | <0.0001 | −0.21 | 0.06 |
| 0–2000 | −0.46 | <0.0001 | −0.44 | <0.0001 | −0.49 | <0.0001 | −0.20 | 0.09 |
| 0–2500 | −0.41 | 0.0002 | −0.40 | 0.0003 | −0.42 | 0.0002 | −0.28 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporale, A.S.; Nezzo, M.; Di Trani, M.G.; Maiuro, A.; Miano, R.; Bove, P.; Mauriello, A.; Manenti, G.; Capuani, S. Acquisition Parameters Influence Diffusion Metrics Effectiveness in Probing Prostate Tumor and Age-Related Microstructure. J. Pers. Med. 2023, 13, 860. https://doi.org/10.3390/jpm13050860
Caporale AS, Nezzo M, Di Trani MG, Maiuro A, Miano R, Bove P, Mauriello A, Manenti G, Capuani S. Acquisition Parameters Influence Diffusion Metrics Effectiveness in Probing Prostate Tumor and Age-Related Microstructure. Journal of Personalized Medicine. 2023; 13(5):860. https://doi.org/10.3390/jpm13050860
Chicago/Turabian StyleCaporale, Alessandra Stella, Marco Nezzo, Maria Giovanna Di Trani, Alessandra Maiuro, Roberto Miano, Pierluigi Bove, Alessandro Mauriello, Guglielmo Manenti, and Silvia Capuani. 2023. "Acquisition Parameters Influence Diffusion Metrics Effectiveness in Probing Prostate Tumor and Age-Related Microstructure" Journal of Personalized Medicine 13, no. 5: 860. https://doi.org/10.3390/jpm13050860