Sympathetic Modulation in Cardiac Arrhythmias: Where We Stand and Where We Go
Abstract
:1. Introduction
2. Neuroanatomy of the Sympathetic System
3. Assessing Autonomic Nervous System (ANS) Function
3.1. Heart Rate Variability (HRV)
3.2. Baroreflex Sensitivity
3.3. Heart Rate Turbulence (HRT)
3.4. T-Wave Alternans (TWA)
4. Situational Conditions Related to Sympathoexcitation and Ventricular Arrhythmias
5. Sympathoexcitation and Regional Electrical Property Modulation
6. Sympathetic Remodeling after Myocardial Infarction
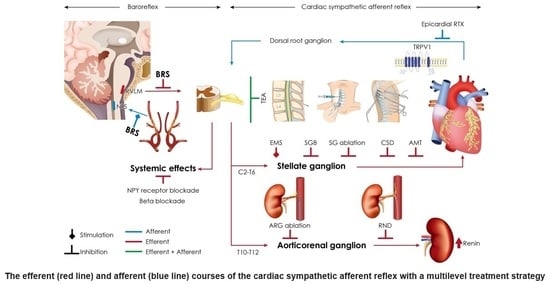
7. Clinical Implications
7.1. Transepidural Anesthesia (TEA)
7.2. Renal Nerve Denervation (RND)
7.3. Cardiac Sympathetic Denervation (CSD) [72]
7.4. Stellate Ganglion Block (SGB)
7.5. Carotid Baroreceptor Stimulation
7.6. Subcutaneous Nerve Stimulation (ScNS)
8. Future Directions
8.1. Pharmacological Sympathetic Modulation
8.2. Aortorenal Ganglion (ARG) Ablation
8.3. Resiniferatoxin (RTX) Deafferentation
8.4. Modified CSD
8.5. Axonal Modulation Therapy (AMT)
8.6. Magnetic and Ultrasound Stimulation
8.7. Glial Cell Modulation
| Interventions | Indication | Mechanisms | Current Implementation | Notes |
|---|---|---|---|---|
| Pharmacological | ||||
| Beta-blocker (BB) | VA, SCD, and heart failure | Inhibits sympathetic effects by blocking BB | Well established | Some patients have VAs despite maximal doses. |
| NPY-receptor blocker [101] | Inhibit sympathoexcitation | Inhibits sympathetic effects by blocking NPY receptors (BIBO3304) | Acute large animal study |
|
| Stellate ganglion modulation | ||||
| CSD via endoscopy assistance [77] | Refractory VA in Long QT, CPVT, ICM, and NICM | Disrupting neural transmission through SG by removing the sympathetic chain and SG | Survival benefit in multileft retrospective studies |
|
| Modified CSD | ||||
| Radiofrequency ablation of the sympathetic chain [107] | Refractory VA In mixed etiology | Disrupting sympathetic chain neural transmission by radiofrequency ablation | 1 clinical case series |
|
| Cryothermal ablation [112] | ES | Disrupting sympathetic chain neural transmission by cryothermal damage to the cervical SG | 1 case report | Has therapeutic potential. |
| Magnetic stimulation of SG [121,122] | ES | Long-term depression and long-term potentiation change synaptic plasticity | Case series of 5 Acute large animal model | No histology or electrophysiology data. |
| Ultrasound stimulation of SG [119] | VA | Unknown mechanism. Possibly through thermal effects and anti-inflammatory effects | Acute large animal model | Unknown mechanism. |
| Stellate ganglion block (SGB) [82] | ES, bailed-out therapy Pain | Pharmacological temporal inhibition of cervical stellate ganglia nerve transmission to the myocardium |
|
|
| Transepidural anesthesia [59] | ES, bailed-out therapy Pain | Pharmacological temporal inhibition of cardiac afferents and sympathetic efferents at levels C8–T4 |
|
|
| Therapies beyond the cervicothoracic SG and sympathetic chain | ||||
| Renal nerve denervation [64] |
| Disrupting sympathetic nerves to modulate the circulating catecholamine release |
|
|
| Aorticorenal ganglion (ARG) ablation [103] |
| Ablating the ganglion is more effective than ablating the renal nerves. |
|
|
| Low-level baroreceptor stimulation [86] |
| By activating the afferents, the parasympathetic efferents are stimulated and the sympathetic efferents are inhibited. |
|
|
| Epicardial RTX deafferentation [106] |
| Selectively and irreversibly activates the TRPV1 channel and then blocks the cardiac afferent reflex. |
|
|
| Axonal modulation therapy [118] |
| KHFAC blocks action potential propagation |
|
|
| Glial cell modulation [120] |
| A designer drug (DRE-ADD) can cause sympathoexcitation by activating SG glial cells | Acute small animal study (Proof-of-concept) |
|
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graboys, T.B. Stress and the aching heart. N. Engl. J. Med. 1984, 311, 594–595. [Google Scholar] [CrossRef]
- Goldberger, J.J.; Arora, R.; Buckley, U.; Shivkumar, K. Autonomic Nervous System Dysfunction: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1189–1206. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Choi, E.K.; Tan, A.Y.; Lin, S.F.; Fishbein, M.C.; Chen, L.S.; Chen, P.S. Neural mechanisms of atrial arrhythmias. Nat. Rev. Cardiol. 2011, 9, 30–39. [Google Scholar] [CrossRef]
- Schwartz, P.J. Cutting nerves and saving lives. Heart Rhythm. 2009, 6, 760–763. [Google Scholar] [CrossRef]
- Yanowitz, F.; Preston, J.B.; Abildskov, J.A. Functional distribution of right and left stellate innervation to the ventricles. Production of neurogenic electrocardiographic changes by unilateral alteration of sympathetic tone. Circ. Res. 1966, 18, 416–428. [Google Scholar] [CrossRef]
- Cao, J.M.; Fishbein, M.C.; Han, J.B.; Lai, W.W.; Lai, A.C.; Wu, T.J.; Czer, L.; Wolf, P.L.; Denton, T.A.; Shintaku, I.P.; et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 2000, 101, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.B.; Wu, C.C.; Lu, L.S.; Su, M.J.; Lin, C.W.; Lin, S.F.; Chen, L.S.; Fishbein, M.C.; Chen, P.S.; Lee, Y.T. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circ. Res. 2003, 92, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Ardell, J.L.; Armour, J.A. Neurocardiology: Structure-Based Function. Compr. Physiol. 2016, 6, 1635–1653. [Google Scholar] [CrossRef]
- Hadaya, J.; Ardell, J.L. Autonomic Modulation for Cardiovascular Disease. Front. Physiol. 2020, 11, 617459. [Google Scholar] [CrossRef]
- Armour, J.A. Cardiac neuronal hierarchy in health and disease. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R262–R271. [Google Scholar] [CrossRef]
- Angelakos, E.T.; King, M.P.; Millard, R.W. Regional distribution of catecholamines in the hearts of various species. Ann. N. Y. Acad. Sci. 1969, 156, 219–240. [Google Scholar] [CrossRef]
- Esler, M.; Jennings, G.; Lambert, G.; Meredith, I.; Horne, M.; Eisenhofer, G. Overflow of catecholamine neurotransmitters to the circulation: Source, fate, and functions. Physiol. Rev. 1990, 70, 963–985. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, B.W.; Kitney, R.I.; Sayers, B.M. Spontaneous rhythms in physiological control systems. Nature 1971, 233, 339–341. [Google Scholar] [CrossRef]
- Pagani, M.; Lombardi, F.; Guzzetti, S.; Rimoldi, O.; Furlan, R.; Pizzinelli, P.; Sandrone, G.; Malfatto, G.; Dell’Orto, S.; Piccaluga, E.; et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ. Res. 1986, 59, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Sayers, B.M. Analysis of heart rate variability. Ergonomics 1973, 16, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.D.; Saul, J.P.; Cohen, R.J. Transfer function analysis of autonomic regulation. I. Canine atrial rate response. Am. J. Physiol. 1989, 256, H142–H152. [Google Scholar] [CrossRef]
- Rahman, F.; Pechnik, S.; Gross, D.; Sewell, L.; Goldstein, D.S. Low frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Clin. Auton. Res. 2011, 21, 133–141. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Vanoli, E.; Stramba-Badiale, M.; De Ferrari, G.M.; Billman, G.E.; Foreman, R.D. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation 1988, 78, 969–979. [Google Scholar] [CrossRef]
- Mortara, A.; La Rovere, M.T.; Pinna, G.D.; Prpa, A.; Maestri, R.; Febo, O.; Pozzoli, M.; Opasich, C.; Tavazzi, L. Arterial baroreflex modulation of heart rate in chronic heart failure: Clinical and hemodynamic correlates and prognostic implications. Circulation 1997, 96, 3450–3458. [Google Scholar] [CrossRef]
- Pinna, G.D.; Maestri, R.; Capomolla, S.; Febo, O.; Robbi, E.; Cobelli, F.; La Rovere, M.T. Applicability and clinical relevance of the transfer function method in the assessment of baroreflex sensitivity in heart failure patients. J. Am. Coll. Cardiol. 2005, 46, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.; Malik, M.; Barthel, P.; Schneider, R.; Ulm, K.; Rolnitzky, L.; Camm, A.J.; Bigger, J.T., Jr.; Schomig, A. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet 1999, 353, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E.; Schwartz, P.J.; Stone, H.L. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation 1984, 69, 1182–1189. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.S.; Jackson, L.E.; Smith, J.M.; Garan, H.; Ruskin, J.N.; Cohen, R.J. Electrical alternans and vulnerability to ventricular arrhythmias. N. Engl. J. Med. 1994, 330, 235–241. [Google Scholar] [CrossRef]
- Ikeda, T.; Yoshino, H.; Sugi, K.; Tanno, K.; Shimizu, H.; Watanabe, J.; Kasamaki, Y.; Yoshida, A.; Kato, T. Predictive value of microvolt T-wave alternans for sudden cardiac death in patients with preserved cardiac function after acute myocardial infarction: Results of a collaborative cohort study. J. Am. Coll. Cardiol. 2006, 48, 2268–2274. [Google Scholar] [CrossRef]
- Skinner, J.E.; Reed, J.C. Blockade of frontocortical-brain stem pathway prevents ventricular fibrillation of ischemic heart. Am. J. Physiol. 1981, 240, H156–H163. [Google Scholar] [CrossRef]
- Lampert, R.; Shusterman, V.; Burg, M.; McPherson, C.; Batsford, W.; Goldberg, A.; Soufer, R. Anger-induced T-wave alternans predicts future ventricular arrhythmias in patients with implantable cardioverter-defibrillators. J. Am. Coll. Cardiol. 2009, 53, 774–778. [Google Scholar] [CrossRef]
- Taggart, P.; Sutton, P.; Redfern, C.; Batchvarov, V.N.; Hnatkova, K.; Malik, M.; James, U.; Joseph, A. The effect of mental stress on the non-dipolar components of the T wave: Modulation by hypnosis. Psychosom. Med. 2005, 67, 376–383. [Google Scholar] [CrossRef]
- Lampert, R.; Shusterman, V.; Burg, M.M.; Lee, F.A.; Earley, C.; Goldberg, A.; McPherson, C.A.; Batsford, W.P.; Soufer, R. Effects of psychologic stress on repolarization and relationship to autonomic and hemodynamic factors. J. Cardiovasc. Electrophysiol. 2005, 16, 372–377. [Google Scholar] [CrossRef]
- Zipes, D.P.; Rubart, M. Neural modulation of cardiac arrhythmias and sudden cardiac death. Heart Rhythm. 2006, 3, 108–113. [Google Scholar] [CrossRef]
- La Rovere, M.T.; Bigger, J.T., Jr.; Marcus, F.I.; Mortara, A.; Schwartz, P.J. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes after Myocardial Infarction) Investigators. Lancet 1998, 351, 478–484. [Google Scholar] [CrossRef]
- Craig, A.D. Forebrain emotional asymmetry: A neuroanatomical basis? Trends. Cogn. Sci. 2005, 9, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.; Thompson, D.R.; Oldridge, N.; Zwisler, A.D.; Rees, K.; Martin, N.; Taylor, R.S. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Database Syst. Rev. 2016, 2016, CD001800. [Google Scholar] [CrossRef] [PubMed]
- Howden, E.J.; Sarma, S.; Lawley, J.S.; Opondo, M.; Cornwell, W.; Stoller, D.; Urey, M.A.; Adams-Huet, B.; Levine, B.D. Reversing the Cardiac Effects of Sedentary Aging in Middle Age-A Randomized Controlled Trial: Implications For Heart Failure Prevention. Circulation 2018, 137, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Qin, R.; Murakoshi, N.; Xu, D.; Tajiri, K.; Feng, D.; Stujanna, E.N.; Yonebayashi, S.; Nakagawa, Y.; Shimano, H.; Nogami, A.; et al. Exercise training reduces ventricular arrhythmias through restoring calcium handling and sympathetic tone in myocardial infarction mice. Physiol. Rep. 2019, 7, e13972. [Google Scholar] [CrossRef] [PubMed]
- Rolim, N.; Skardal, K.; Hoydal, M.; Sousa, M.M.; Malmo, V.; Kaurstad, G.; Ingul, C.B.; Hansen, H.E.; Alves, M.N.; Thuen, M.; et al. Aerobic interval training reduces inducible ventricular arrhythmias in diabetic mice after myocardial infarction. Basic Res. Cardiol. 2015, 110, 44. [Google Scholar] [CrossRef] [PubMed]
- Raukar, N.; Arciero, E.; Noyes, A.; Drezner, J.; Weiss, J. Cardiovascular pre-participation screening in the young athlete: Addressing concerns. Phys. Sportsmed. 2017, 45, 365–369. [Google Scholar] [CrossRef]
- Polyak, A.; Topal, L.; Zombori-Toth, N.; Toth, N.; Prorok, J.; Kohajda, Z.; Deri, S.; Demeter-Haludka, V.; Hegyi, P.; Venglovecz, V.; et al. Cardiac electrophysiological remodeling associated with enhanced arrhythmia susceptibility in a canine model of elite exercise. Elife 2023, 12, e80710. [Google Scholar] [CrossRef]
- Shení1 Zipes, D.P. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef]
- Bers, D.M. Cardiac excitation-contraction coupling. Nature 2002, 415, 198–205. [Google Scholar] [CrossRef]
- Bers, D.M. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008, 70, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.O.; Kurokawa, J.; Reiken, S.; Motoike, H.; D’Armiento, J.; Marks, A.R.; Kass, R.S. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 2002, 295, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Wallace, A.G.; Sarnoff, S.J. Effects of Cardiac Sympathetic Nerve Stimulation on Conduction in the Heart. Circ. Res. 1964, 14, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, M.; Yamakawa, K.; Sinha, A.; So, E.L.; Zhou, W.; Ajijola, O.A.; Lux, R.L.; Laks, M.; Shivkumar, K.; Mahajan, A. Modulation of regional dispersion of repolarization and T-peak to T-end interval by the right and left stellate ganglia. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1020–H1030. [Google Scholar] [CrossRef] [PubMed]
- Kember, G.; Armour, J.A.; Zamir, M. Neural control hierarchy of the heart has not evolved to deal with myocardial ischemia. Physiol. Genom. 2013, 45, 638–644. [Google Scholar] [CrossRef]
- Zucker, I.H.; Patel, K.P.; Schultz, H.D. Neurohumoral stimulation. Heart Fail Clin. 2012, 8, 87–99. [Google Scholar] [CrossRef]
- Inoue, H.; Zipes, D.P. Time course of denervation of efferent sympathetic and vagal nerves after occlusion of the coronary artery in the canine heart. Circ. Res. 1988, 62, 1111–1120. [Google Scholar] [CrossRef]
- Zipes, D.P.; Barber, M.J.; Takahashi, N.; Gilmour, R.F., Jr. Influence of the autonomic nervous system on the genesis of cardiac arrhythmias. Pacing. Clin. Electrophysiol. 1983, 6, 1210–1220. [Google Scholar] [CrossRef]
- Parthenakis, F.I.; Prassopoulos, V.K.; Koukouraki, S.I.; Zacharis, E.A.; Diakakis, G.F.; Karkavitsas, N.K.; Vardas, P.E. Segmental pattern of myocardial sympathetic denervation in idiopathic dilated cardiomyopathy: Relationship to regional wall motion and myocardial perfusion abnormalities. J. Nucl. Cardiol. 2002, 9, 15–22. [Google Scholar] [CrossRef]
- Rabinovitch, M.A.; Rose, C.P.; Rouleau, J.L.; Chartrand, C.; Wieland, D.M.; Lepanto, L.; Legault, F.; Suissa, S.; Rosenthall, L.; Burgess, J.H. Metaiodobenzylguanidine [131I] scintigraphy detects impaired myocardial sympathetic neuronal transport function of canine mechanical-overload heart failure. Circ. Res. 1987, 61, 797–804. [Google Scholar] [CrossRef]
- Fu, S.Y.; Gordon, T. The cellular and molecular basis of peripheral nerve regeneration. Mol. Neurobiol. 1997, 14, 67–116. [Google Scholar] [CrossRef]
- Cha, Y.M.; Redfield, M.M.; Shah, S.; Shen, W.K.; Fishbein, M.C.; Chen, P.S. Effects of omapatrilat on cardiac nerve sprouting and structural remodeling in experimental congestive heart failure. Heart Rhythm. 2005, 2, 984–990. [Google Scholar] [CrossRef]
- Kanazawa, H.; Ieda, M.; Kimura, K.; Arai, T.; Kawaguchi-Manabe, H.; Matsuhashi, T.; Endo, J.; Sano, M.; Kawakami, T.; Kimura, T.; et al. Heart failure causes cholinergic transdifferentiation of cardiac sympathetic nerves via gp130-signaling cytokines in rodents. J. Clin. Investig. 2010, 120, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.J.; Shinohara, T.; Park, H.W.; Frick, K.; Ice, D.S.; Choi, E.K.; Han, S.; Maruyama, M.; Sharma, R.; Shen, C.; et al. Continuous low-level vagus nerve stimulation reduces stellate ganglion nerve activity and paroxysmal atrial tachyarrhythmias in ambulatory canines. Circulation 2011, 123, 2204–2212. [Google Scholar] [CrossRef] [PubMed]
- Ajijola, O.A.; Yagishita, D.; Reddy, N.K.; Yamakawa, K.; Vaseghi, M.; Downs, A.M.; Hoover, D.B.; Ardell, J.L.; Shivkumar, K. Remodeling of stellate ganglion neurons after spatially targeted myocardial infarction: Neuropeptide and morphologic changes. Heart Rhythm. 2015, 12, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A.; Eckardt, L.; Kirchhof, P.; Weber, T.; Rolf, N.; Breithardt, G.; Van Aken, H.; Haverkamp, W. Effects of thoracic epidural anesthesia with and without autonomic nervous system blockade on cardiac monophasic action potentials and effective refractoriness in awake dogs. Anesthesiology 2001, 95, 132–138; discussion 136A. [Google Scholar] [CrossRef]
- Bourke, T.; Vaseghi, M.; Michowitz, Y.; Sankhla, V.; Shah, M.; Swapna, N.; Boyle, N.G.; Mahajan, A.; Narasimhan, C.; Lokhandwala, Y.; et al. Neuraxial modulation for refractory ventricular arrhythmias: Value of thoracic epidural anesthesia and surgical left cardiac sympathetic denervation. Circulation 2010, 121, 2255–2262. [Google Scholar] [CrossRef]
- Wink, J.; Steendijk, P.; Tsonaka, R.; de Wilde, R.B.P.; Friedericy, H.J.; Braun, J.; Veering, B.T.; Aarts, L.; Wouters, P.F. Biventricular function in exercise during autonomic (thoracic epidural) block. Eur. J. Appl. Physiol. 2021, 121, 1405–1418. [Google Scholar] [CrossRef]
- Do, D.H.; Bradfield, J.; Ajijola, O.A.; Vaseghi, M.; Le, J.; Rahman, S.; Mahajan, A.; Nogami, A.; Boyle, N.G.; Shivkumar, K. Thoracic Epidural Anesthesia Can Be Effective for the Short-Term Management of Ventricular Tachycardia Storm. J. Am. Heart Assoc. 2017, 6, e007080. [Google Scholar] [CrossRef]
- Azizi, M.; Schmieder, R.E.; Mahfoud, F.; Weber, M.A.; Daemen, J.; Davies, J.; Basile, J.; Kirtane, A.J.; Wang, Y.; Lobo, M.D.; et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): A multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018, 391, 2335–2345. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Shabanov, V.; Ponomarev, D.; Losik, D.; Ivanickiy, E.; Kropotkin, E.; Polyakov, K.; Ptaszynski, P.; Keweloh, B.; Yao, C.J.; et al. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA 2020, 323, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Ukena, C.; Bauer, A.; Mahfoud, F.; Schreieck, J.; Neuberger, H.R.; Eick, C.; Sobotka, P.A.; Gawaz, M.; Bohm, M. Renal sympathetic denervation for treatment of electrical storm: First-in-man experience. Clin. Res. Cardiol. 2012, 101, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Evranos, B.; Canpolat, U.; Kocyigit, D.; Coteli, C.; Yorgun, H.; Aytemir, K. Role of Adjuvant Renal Sympathetic Denervation in the Treatment of Ventricular Arrhythmias. Am. J. Cardiol. 2016, 118, 1207–1210. [Google Scholar] [CrossRef]
- Bradfield, J.S.; Hayase, J.; Liu, K.; Moriarty, J.; Kee, S.T.; Do, D.; Ajijola, O.A.; Vaseghi, M.; Gima, J.; Sorg, J.; et al. Renal denervation as adjunctive therapy to cardiac sympathetic denervation for ablation refractory ventricular tachycardia. Heart Rhythm. 2020, 17, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Huang, B.; Zhou, X.; Wang, S.; Wang, Z.; Wang, M.; Li, X.; Zhou, L.; Meng, G.; Yuan, S.; et al. Renal sympathetic stimulation and ablation affect ventricular arrhythmia by modulating autonomic activity in a cesium-induced long QT canine model. Heart Rhythm. 2017, 14, 912–919. [Google Scholar] [CrossRef]
- Tsai, W.C.; Chan, Y.H.; Chinda, K.; Chen, Z.; Patel, J.; Shen, C.; Zhao, Y.; Jiang, Z.; Yuan, Y.; Ye, M.; et al. Effects of renal sympathetic denervation on the stellate ganglion and brain stem in dogs. Heart Rhythm. 2017, 14, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Linz, D.; Ukena, C.; Esler, M.; Mahfoud, F. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond? Circ. Res. 2014, 115, 400–409. [Google Scholar] [CrossRef]
- Mulder, J.; Hokfelt, T.; Knuepfer, M.M.; Kopp, U.C. Renal sensory and sympathetic nerves reinnervate the kidney in a similar time-dependent fashion after renal denervation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R675–R682. [Google Scholar] [CrossRef]
- Couch, N.P.; Mc, B.R.; Dammin, G.J.; Murray, J.E. Observations on the nature of the enlargement, the regeneration of the nerves, and the function of the canine renal autograft. Br. J. Exp. Pathol. 1961, 42, 106–113. [Google Scholar]
- Booth, L.C.; Nishi, E.E.; Yao, S.T.; Ramchandra, R.; Lambert, G.W.; Schlaich, M.P.; May, C.N. Reinnervation following catheter-based radio-frequency renal denervation. Exp. Physiol. 2015, 100, 485–490. [Google Scholar] [CrossRef]
- Qian, P.C.; Barry, M.A.; Lu, J.; Pouliopoulos, J.; Mina, A.; Bandodkar, S.; Alvarez, S.; James, V.; Ronquillo, J.; Varikatt, W.; et al. Transvascular Pacing of Aorticorenal Ganglia Provides a Testable Procedural Endpoint for Renal Artery Denervation. JACC Cardiovasc. Interv. 2019, 12, 1109–1120. [Google Scholar] [CrossRef]
- Yalin, K.; Liosis, S.; Palade, E.; Fink, T.; Schierholz, S.; Sawan, N.; Eitel, C.; Heeger, C.H.; Sciacca, V.; Sano, M.; et al. Cardiac sympathetic denervation in patients with nonischemic cardiomyopathy and refractory ventricular arrhythmias: A single-center experience. Clin. Res. Cardiol. 2021, 110, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Foreman, R.D.; Stone, H.L.; Brown, A.M. Effect of dorsal root section on the arrhythmias associated with coronary occlusion. Am. J. Physiol. 1976, 231, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Yamakawa, K.; Hamon, D.; Nakamura, K.; Shivkumar, K.; Vaseghi, M. Cardiac sympathetic innervation via middle cervical and stellate ganglia and antiarrhythmic mechanism of bilateral stellectomy. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H392–H405. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.J.; Priori, S.G.; Cerrone, M.; Spazzolini, C.; Odero, A.; Napolitano, C.; Bloise, R.; De Ferrari, G.M.; Klersy, C.; Moss, A.J.; et al. Left cardiac sympathetic denervation in the management of high-risk patients affected by the long-QT syndrome. Circulation 2004, 109, 1826–1833. [Google Scholar] [CrossRef]
- De Ferrari, G.M.; Dusi, V.; Spazzolini, C.; Bos, J.M.; Abrams, D.J.; Berul, C.I.; Crotti, L.; Davis, A.M.; Eldar, M.; Kharlap, M.; et al. Clinical Management of Catecholaminergic Polymorphic Ventricular Tachycardia: The Role of Left Cardiac Sympathetic Denervation. Circulation 2015, 131, 2185–2193. [Google Scholar] [CrossRef]
- Vaseghi, M.; Gima, J.; Kanaan, C.; Ajijola, O.A.; Marmureanu, A.; Mahajan, A.; Shivkumar, K. Cardiac sympathetic denervation in patients with refractory ventricular arrhythmias or electrical storm: Intermediate and long-term follow-up. Heart Rhythm. 2014, 11, 360–366. [Google Scholar] [CrossRef]
- Della Bella, P.; Baratto, F.; Tsiachris, D.; Trevisi, N.; Vergara, P.; Bisceglia, C.; Petracca, F.; Carbucicchio, C.; Benussi, S.; Maisano, F.; et al. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: Long-term outcome after ablation. Circulation 2013, 127, 1359–1368. [Google Scholar] [CrossRef]
- Shah, R.; Assis, F.; Alugubelli, N.; Okada, D.R.; Cardoso, R.; Shivkumar, K.; Tandri, H. Cardiac sympathetic denervation for refractory ventricular arrhythmias in patients with structural heart disease: A systematic review. Heart Rhythm. 2019, 16, 1499–1505. [Google Scholar] [CrossRef]
- Ackerman, W.E.; Zhang, J.M. Efficacy of stellate ganglion blockade for the management of type 1 complex regional pain syndrome. South Med. J. 2006, 99, 1084–1088. [Google Scholar] [CrossRef]
- Tian, Y.; Wittwer, E.D.; Kapa, S.; McLeod, C.J.; Xiao, P.; Noseworthy, P.A.; Mulpuru, S.K.; Deshmukh, A.J.; Lee, H.C.; Ackerman, M.J.; et al. Effective Use of Percutaneous Stellate Ganglion Blockade in Patients With Electrical Storm. Circ. Arrhythm. Electrophysiol. 2019, 12, e007118. [Google Scholar] [CrossRef] [PubMed]
- Fudim, M.; Qadri, Y.J.; Waldron, N.H.; Boortz-Marx, R.L.; Ganesh, A.; Patel, C.B.; Podgoreanu, M.V.; Sun, A.Y.; Milano, C.A.; Tong, B.C.; et al. Stellate Ganglion Blockade for the Treatment of Refractory Ventricular Arrhythmias. JACC Clin. Electrophysiol. 2020, 6, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.F.; Tarikci Kilic, E.; Aksoy, Y.; Kaydu, A.; Gokcek, E. The importance of perfusion index monitoring in evaluating the efficacy of stellate ganglion blockage treatment in Raynaud’s disease. Libyan J. Med. 2018, 13, 1422666. [Google Scholar] [CrossRef] [PubMed]
- Sanghai, S.; Abbott, N.J.; Dewland, T.A.; Henrikson, C.A.; Elman, M.R.; Wollenberg, M.; Ivie, R.; Gonzalez-Sotomayor, J.; Nazer, B. Stellate Ganglion Blockade With Continuous Infusion Versus Single Injection for Treatment of Ventricular Arrhythmia Storm. JACC Clin. Electrophysiol. 2021, 7, 452–460. [Google Scholar] [CrossRef]
- Schwartz, P.J.; Zaza, A.; Pala, M.; Locati, E.; Beria, G.; Zanchetti, A. Baroreflex sensitivity and its evolution during the first year after myocardial infarction. J. Am. Coll. Cardiol. 1988, 12, 629–636. [Google Scholar] [CrossRef]
- Liao, K.; Yu, L.; He, B.; Huang, B.; Yang, K.; Saren, G.; Wang, S.; Zhou, X.; Jiang, H. Carotid baroreceptor stimulation prevents arrhythmias induced by acute myocardial infarction through autonomic modulation. J. Cardiovasc. Pharmacol. 2014, 64, 431–437. [Google Scholar] [CrossRef]
- Zile, M.R.; Lindenfeld, J.; Weaver, F.A.; Zannad, F.; Galle, E.; Rogers, T.; Abraham, W.T. Baroreflex Activation Therapy in Patients With Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2020, 76, 1–13. [Google Scholar] [CrossRef]
- Yuan, Y.; Jiang, Z.; Zhao, Y.; Tsai, W.C.; Patel, J.; Chen, L.S.; Shen, C.; Lin, S.F.; Chen, H.V.; Everett, T.H.t.; et al. Long-term intermittent high-amplitude subcutaneous nerve stimulation reduces sympathetic tone in ambulatory dogs. Heart Rhythm. 2018, 15, 451–459. [Google Scholar] [CrossRef]
- Kusayama, T.; Wan, J.; Yuan, Y.; Liu, X.; Li, X.; Shen, C.; Fishbein, M.C.; Everett, T.H.t.; Chen, P.S. Effects of subcutaneous nerve stimulation with blindly inserted electrodes on ventricular rate control in a canine model of persistent atrial fibrillation. Heart Rhythm. 2021, 18, 261–270. [Google Scholar] [CrossRef]
- Swedberg, K.; Komajda, M.; Bohm, M.; Borer, J.S.; Ford, I.; Dubost-Brama, A.; Lerebours, G.; Tavazzi, L.; Investigators, S. Ivabradine and outcomes in chronic heart failure (SHIFT): A randomised placebo-controlled study. Lancet 2010, 376, 875–885. [Google Scholar] [CrossRef]
- Taub, P.R.; Zadourian, A.; Lo, H.C.; Ormiston, C.K.; Golshan, S.; Hsu, J.C. Randomized Trial of Ivabradine in Patients With Hyperadrenergic Postural Orthostatic Tachycardia Syndrome. J. Am. Coll. Cardiol. 2021, 77, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Cappato, R.; Castelvecchio, S.; Ricci, C.; Bianco, E.; Vitali-Serdoz, L.; Gnecchi-Ruscone, T.; Pittalis, M.; De Ambroggi, L.; Baruscotti, M.; Gaeta, M.; et al. Clinical efficacy of ivabradine in patients with inappropriate sinus tachycardia: A prospective, randomized, placebo-controlled, double-blind, crossover evaluation. J. Am. Coll. Cardiol. 2012, 60, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Bohm, M.; Borer, J.S.; Camm, J.; Ford, I.; Lloyd, S.M.; Komajda, M.; Tavazzi, L.; Talajic, M.; Lainscak, M.; Reil, J.C.; et al. Twenty-four-hour heart rate lowering with ivabradine in chronic heart failure: Insights from the SHIFT Holter substudy. Eur. J. Heart Fail. 2015, 17, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Scridon, A.; Halatiu, V.B.; Balan, A.I.; Cozac, D.A.; Moldovan, V.; Banescu, C.; Perian, M.; Serban, R.C. Long-Term Effects of Ivabradine on Cardiac Vagal Parasympathetic Function in Normal Rats. Front. Pharmacol. 2021, 12, 596956. [Google Scholar] [CrossRef] [PubMed]
- Kawada, T.; Shimizu, S.; Uemura, K.; Hayama, Y.; Yamamoto, H.; Shishido, T.; Nishikawa, T.; Sugimachi, M. Ivabradine preserves dynamic sympathetic control of heart rate despite inducing significant bradycardia in rats. J. Physiol. Sci. 2019, 69, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Herring, N.; Lokale, M.N.; Danson, E.J.; Heaton, D.A.; Paterson, D.J. Neuropeptide Y reduces acetylcholine release and vagal bradycardia via a Y2 receptor-mediated, protein kinase C-dependent pathway. J. Mol. Cell. Cardiol. 2008, 44, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Heredia Mdel, P.; Delgado, C.; Pereira, L.; Perrier, R.; Richard, S.; Vassort, G.; Benitah, J.P.; Gomez, A.M. Neuropeptide Y rapidly enhances [Ca2+]i transients and Ca2+ sparks in adult rat ventricular myocytes through Y1 receptor and PLC activation. J. Mol. Cell. Cardiol. 2005, 38, 205–212. [Google Scholar] [CrossRef]
- Parker, E.; Van Heek, M.; Stamford, A. Neuropeptide Y receptors as targets for anti-obesity drug development: Perspective and current status. Eur. J. Pharmacol. 2002, 440, 173–187. [Google Scholar] [CrossRef]
- Ajijola, O.A.; Chatterjee, N.A.; Gonzales, M.J.; Gornbein, J.; Liu, K.; Li, D.; Paterson, D.J.; Shivkumar, K.; Singh, J.P.; Herring, N. Coronary Sinus Neuropeptide Y Levels and Adverse Outcomes in Patients With Stable Chronic Heart Failure. JAMA Cardiol. 2020, 5, 318–325. [Google Scholar] [CrossRef]
- Kalla, M.; Hao, G.; Tapoulal, N.; Tomek, J.; Liu, K.; Woodward, L.; Oxford Acute Myocardial Infarction, S.; Dall’Armellina, E.; Banning, A.P.; Choudhury, R.P.; et al. The cardiac sympathetic co-transmitter neuropeptide Y is pro-arrhythmic following ST-elevation myocardial infarction despite beta-blockade. Eur. Heart J. 2020, 41, 2168–2179. [Google Scholar] [CrossRef]
- Hoang, J.D.; Salavatian, S.; Yamaguchi, N.; Swid, M.A.; David, H.; Vaseghi, M. Cardiac sympathetic activation circumvents high-dose beta blocker therapy in part through release of neuropeptide Y. JCI Insight 2020, 5. [Google Scholar] [CrossRef] [PubMed]
- Norvell, J.E. The aorticorenal ganglion and its role in renal innervation. J. Comp. Neurol. 1968, 133, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Temma, T.; Wooten, C.; Sobowale, C.; Tahmasian, S.; Chan, C.; Swid, M.A.; Zuckerman, J.E.; Peacock, W.; Ajijola, O.A. Aorticorenal ganglion as a novel target for renal neuromodulation. Heart Rhythm. 2021, 18, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Zahner, M.R.; Li, D.P.; Chen, S.R.; Pan, H.L. Cardiac vanilloid receptor 1-expressing afferent nerves and their role in the cardiogenic sympathetic reflex in rats. J. Physiol. 2003, 551, 515–523. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, W.; Cornish, K.G.; Rozanski, G.J.; Zucker, I.H. Cardiac sympathetic afferent denervation attenuates cardiac remodeling and improves cardiovascular dysfunction in rats with heart failure. Hypertension 2014, 64, 745–755. [Google Scholar] [CrossRef]
- Yoshie, K.; Rajendran, P.S.; Massoud, L.; Mistry, J.; Swid, M.A.; Wu, X.; Sallam, T.; Zhang, R.; Goldhaber, J.I.; Salavatian, S.; et al. Cardiac TRPV1 afferent signaling promotes arrhythmogenic ventricular remodeling after myocardial infarction. JCI Insight 2020, 5, e124477. [Google Scholar] [CrossRef] [PubMed]
- Cauti, F.M.; Rossi, P.; Bianchi, S.; Bruno, K.; Iaia, L.; Rossi, C.; Pugliese, F.; Quaglione, R.; Venuta, F.; Anile, M. Outcome of a Modified Sympathicotomy for Cardiac Neuromodulation of Untreatable Ventricular Tachycardia. JACC Clin. Electrophysiol. 2021, 7, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Assis, F.R.; Sharma, A.; Shah, R.; Akhtar, T.; Adari, S.; Calkins, H.; Ha, J.S.; Mandal, K.; Tandri, H. Long-Term Outcomes of Bilateral Cardiac Sympathetic Denervation for Refractory Ventricular Tachycardia. JACC Clin. Electrophysiol. 2021, 7, 463–470. [Google Scholar] [CrossRef]
- Sekhri, N.K.; Parikh, S.; Foo, R.M. Radiofrequency Ablation of the Stellate Ganglion for Management of Acute Digital Ischemia: A Case Report. A A Pract. 2018, 11, 189–192. [Google Scholar] [CrossRef]
- Roy, C.; Chatterjee, N. Radiofrequency ablation of stellate ganglion in a patient with complex regional pain syndrome. Saudi J. Anaesth. 2014, 8, 408–411. [Google Scholar] [CrossRef]
- Hudec, M.; Jiravsky, O.; Spacek, R.; Neuwirth, R.; Knybel, L.; Sknouril, L.; Cvek, J.; Miklik, R. Chronic refractory angina pectoris treated by bilateral stereotactic radiosurgical stellate ganglion ablation: First-in-man case report. Eur. Heart J. Case Rep. 2021, 5, ytab184. [Google Scholar] [CrossRef] [PubMed]
- Chatzidou, S.; Kontogiannis, C.; Tampakis, K.; Episkopou, E.; Kanakakis, I.; Paraskevaidis, I. Cryoablation of stellate ganglion for the management of electrical storm: The first reported case. Europace 2021, 23, 1105. [Google Scholar] [CrossRef]
- Kilgore, K.L.; Bhadra, N. Reversible nerve conduction block using kilohertz frequency alternating current. Neuromodulation 2014, 17, 242–254; discussion 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Roppolo, J.R.; de Groat, W.C.; Tai, C. Mechanism of nerve conduction block induced by high-frequency biphasic electrical currents. IEEE Trans. Biomed. Eng. 2006, 53, 2445–2454. [Google Scholar] [CrossRef]
- Bhadra, N.; Kilgore, K.L. High-frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve 2005, 32, 782–790. [Google Scholar] [CrossRef]
- Gaunt, R.A.; Prochazka, A. Transcutaneously coupled, high-frequency electrical stimulation of the pudendal nerve blocks external urethral sphincter contractions. Neurorehabil. Neural. Repair. 2009, 23, 615–626. [Google Scholar] [CrossRef]
- Chui, R.W.; Buckley, U.; Rajendran, P.S.; Vrabec, T.; Shivkumar, K.; Ardell, J.L. Bioelectronic block of paravertebral sympathetic nerves mitigates post-myocardial infarction ventricular arrhythmias. Heart Rhythm. 2017, 14, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Hadaya, J.; Buckley, U.; Gurel, N.Z.; Chan, C.A.; Swid, M.A.; Bhadra, N.; Vrabec, T.L.; Hoang, J.D.; Smith, C.; Shivkumar, K.; et al. Scalable and reversible axonal neuromodulation of the sympathetic chain for cardiac control. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H105–H115. [Google Scholar] [CrossRef]
- Wang, S.; Li, B.; Li, X.; Wu, L.; Zhu, T.; Zhao, D.; Jiang, H. Low-Intensity Ultrasound Modulation May Prevent Myocardial Infarction-induced Sympathetic Neural Activation and Ventricular Arrhythmia. J. Cardiovasc. Pharmacol. 2020, 75, 432–438. [Google Scholar] [CrossRef]
- Xie, A.X.; Lee, J.J.; McCarthy, K.D. Ganglionic GFAP (+) glial Gq-GPCR signaling enhances heart functions in vivo. JCI Insight 2017, 2, e90565. [Google Scholar] [CrossRef]
- Markman, T.M.; Hamilton, R.H.; Marchlinski, F.E.; Nazarian, S. Case Series of Transcutaneous Magnetic Stimulation for Ventricular Tachycardia Storm. JAMA 2020, 323, 2200–2202. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, M.; Cabrera, A.; Perez, J.O.; de la Rua, J.; Rojas, N.; Zhou, Q.; Pinzon-Ardila, A.; Gonzalez-Arias, S.M.; Adjouadi, M. Induced effects of transcranial magnetic stimulation on the autonomic nervous system and the cardiac rhythm. Sci. World J. 2014, 2014, 349718. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, W.-H.; Lin, Y.-N.; Wu, M.-Y.; Chang, K.-C. Sympathetic Modulation in Cardiac Arrhythmias: Where We Stand and Where We Go. J. Pers. Med. 2023, 13, 786. https://doi.org/10.3390/jpm13050786
Chung W-H, Lin Y-N, Wu M-Y, Chang K-C. Sympathetic Modulation in Cardiac Arrhythmias: Where We Stand and Where We Go. Journal of Personalized Medicine. 2023; 13(5):786. https://doi.org/10.3390/jpm13050786
Chicago/Turabian StyleChung, Wei-Hsin, Yen-Nien Lin, Mei-Yao Wu, and Kuan-Cheng Chang. 2023. "Sympathetic Modulation in Cardiac Arrhythmias: Where We Stand and Where We Go" Journal of Personalized Medicine 13, no. 5: 786. https://doi.org/10.3390/jpm13050786