COPD, but Not Asthma, Is Associated with Worse Outcomes in COVID-19: Real-Life Data from Four Main Centers in Northwest Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diagnosis of COVID-19
2.2. COPD and Asthma Diagnosis
2.3. Comorbidity
2.4. Evaluation of the Hospital Stay
2.5. Statistical Analysis
2.6. Ethical Committee
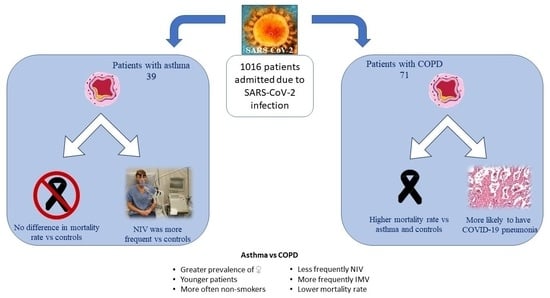
3. Results
3.1. Comparison between Patients with Asthma and Controls
3.2. Comparison between Patients with Asthma and COPD
3.3. Comparison between Asthma Patients without Medications and in Regular ICS
4. Discussion
5. Conclusions
6. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Ashraf, U.M.; Abokor, A.A.; Edwards, J.M. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genom. 2021, 53, 51–60. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Dipalma, G.; Inchingolo, A.M. The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants. Antioxidants 2021, 10, 881. [Google Scholar] [CrossRef]
- Benton, L.D. Childhood Respiratory Conditions: Asthma. FP Essent. 2022, 513, 11–19. [Google Scholar]
- Lokugamage, K.G.; Hage, A.; De Vries, M. Type I Interferon Susceptibility Distinguishes SARS-CoV-2, SARS-CoV. J. Virol. 2020, 9, e01410-20. [Google Scholar] [CrossRef]
- Chang, E.H.; Willis, A.L.; Romanoski, C.E. Rhinovirus Infections in Individuals with Asthma Increase ACE2 Expression and Cytokine Pathways Implicated in COVID-19. Am. J. Respir. Crit. Care Med. 2020, 201, 753–755. [Google Scholar] [CrossRef]
- Puja, R.M.; Semple, G.M.; Shen Lim, W. Predictors of clinical outcome in a national hospitalised cohort across both waves of the influenza A/H1N1 pandemic 2009–2010 in the UK. Thorax 2012, 67, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Coden, M.E.; Loffredo, L.F.; Abdala-Valencia, H. Comparative Study of SARS-CoV-2, SARS-CoV-1, MERS-CoV, HCoV-229E and Influenza Host Gene Expression in Asthma: Importance of Sex, Disease Severity, and Epithelial Heterogeneity. Viruses 2021, 13, 1081. [Google Scholar] [CrossRef]
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; Global Initiative for Asthma: Fontana, WI, USA, 2022. [Google Scholar]
- Braido, F.; Blasi, F.; Canonica, G.W. Mild/Moderate Asthma Network in Italy (MANI): A long-term observational study. J. Asthma 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Antonicelli, L.; Tontini, C.; Manzotti, G. Severe asthma in adults does not significantly affect the outcome of COVID-19 disease: Results from the Italian Severe Asthma Registry. Allergy 2021, 76, 902–905. [Google Scholar] [CrossRef]
- Heffler, E.; Detoraki, A.; Contoli, M. COVID-19 in Severe Asthma Network in Italy (SANI) patients: Clinical features, impact of comorbidities and treatments. Allergy 2021, 76, 887–892. [Google Scholar] [CrossRef]
- Enilari, O.; Sinha, S. The Global Impact of Asthma in Adult Populations. Ann. Glob. Health 2019, 85, 2. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, C.; Gani, F.; Berti, A. Asthma and COVID-19: A dangerous liaison? Asthma Res. Pract. 2021, 7, 9. [Google Scholar] [CrossRef]
- Wang, I.; Wechsler, M.E. Characterization of Severe Asthma Worldwide: Data from the International Severe Asthma Registry. Chest 2020, 157, 790–804. [Google Scholar] [CrossRef]
- Chhiba, K.D.; Patel, G.B.; Vu, T.H.T. Prevalence and characterization of asthma in hospitalized and nonhospitalized patients with COVID-19. J. Allergy Clin. Immunol. 2020, 146, 307–314. [Google Scholar] [CrossRef]
- Saidani, A.; Maddeh, S.; Kallel, N. Is asthma a risk factor for severe outcomes in COVID-19? Eur. Respir. J. 2021, 58 (Suppl. 65), PA2525. [Google Scholar] [CrossRef]
- Peters, U.; Dixon, A.E.; Forno, E. Obesity and asthma. J. Allergy Clin. Immunol. 2018, 141, 1169–1179. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance (accessed on 7 June 2022).
- Global Initiative for Chronic Obstructive Lung Disease. The Global Strategy for Diagnosis, Management and Prevention of COPD 2022 Report; Global Initiative for Chronic Obstructive Lung Disease: Fontana, WI, USA, 2022. [Google Scholar]
- Cheng, L.I.; Rascati, K.L. Validation of the updated charlson comorbidity index (cci) for use in patients with diabetes or asthma: A comparison study. Value Health 2011, 14, A233–A510. [Google Scholar] [CrossRef] [Green Version]
- Spagnuolo, V.; Guffanti, M.; Galli, L.; COVID-BioB Study Group. Viral clearance after early corticosteroid treatment in patients with moderate or severe COVID-19. Sci. Rep. 2020, 10, 21291. [Google Scholar] [CrossRef]
- Prediletto, I.; D’Antoni, L.; Carbonara, P. Standardising PaO2 for PaCO2 in P/F ratio predicts in-hospital mortality in acute respiratory failure due to COVID-19: A pilot prospective study. Eur. J. Intern. Med. 2021, 92, 48–54. [Google Scholar] [CrossRef]
- Korkmaz, İ.; Keleş, F. COVID-19-Related Lung Involvement at Different Time Intervals: Evaluation of Computed Tomography Images with Semiquantitative Scoring System and COVID-19 Reporting and Data System Scoring. Cureus 2021, 13, e18554. [Google Scholar] [CrossRef]
- Myall, K.J.; Martinovic, J.L.; West, A. How COVID-19 interacts with interstitial lung disease. Breathe 2022, 18, 210158. [Google Scholar] [CrossRef] [PubMed]
- Wasilewski, P.G.; Mruk, B.; Mazur, S. COVID-19 severity scoring systems in radiological imaging—A review. Pol. J. Radiol. 2020, 85, 361–368. [Google Scholar] [CrossRef] [PubMed]
- CIRCOLARE SULLE PROCEDURE SEMPLIFICATE PER GLI STUDI E I PROGRAMMI DI USO TERAPEUTICOCOMPASSIONEVOLE PER L’EMERGENZA DA COVID-19. Available online: https://www.aifa.gov.it/documents/20142/1123276/CIRCOLARE_ART-40_studi_programmi_COVI-19_22.05.2020.pdf/ca6d36a9-caa6-9ad4-31fc-4e44acae480d (accessed on 16 May 2022).
- Liu, S.; Cao, Y.; Du, T. Prevalence of Comorbid Asthma and Related Outcomes in COVID-19: A Systematic Review and Meta-Analysis. J. Allergy Clin. Immunol. Pract. 2021, 9, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Çölkesen, F.; Kılınçel, O.; Sözen, M. The impact of SARS-CoV-2 transmission fear and COVID-19 pandemic on the mental health of patients with primary immunodeficiency disorders, severe asthma, and other high-risk groups. Asthma Allergy Immunol. 2021, 19, 84–91. [Google Scholar] [CrossRef]
- Adir, Y.; Humbert, M.; Saliba, W. COVID-19 risk and outcomes in adult asthmatic patients treated with biologics or systemic corticosteroids: Nationwide real-world evidence. J. Allergy Clin. Immunol. 2021, 148, 361–367. [Google Scholar] [CrossRef]
- Solidoro, P.; Nicola, S.; Ridolfi, I. Biologics in Severe Eosinophilic Asthma: Three-Year Follow-Up in a SANI Single Center. Biomedicines 2022, 10, 200. [Google Scholar] [CrossRef]
- Bagnasco, D.; Brussino, L.; Caruso, C. Do the current guidelines for asthma pharmacotherapy encourage over-treatment? Expert Opin. Pharmacother. 2020, 21, 1283–1286. [Google Scholar] [CrossRef]
- Tabassum, T.; Rahman, A.; Araf, Y. Management of asthma patients during the COVID-19 pandemic: Pathophysiological considerations to address the challenges. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 20. [Google Scholar] [CrossRef]
- Kaye, L.; Theye, B.; Smeenk, I. Changes in medication adherence among patients with asthma and COPD during the COVID-19 pandemic. J. Allergy Clin. Immunol. Pract. 2020, 8, 2384–2385. [Google Scholar] [CrossRef]
- Finney, L.J.; Glanville, N.; Farne, H. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J. Allergy Clin. Immunol. 2021, 147, 510–519. [Google Scholar] [CrossRef]
- Giorgis, V.; Rolla, G.; Raie, A. A Case of Work-Related Donkey Milk Allergy. J. Investig. Allergol. Clin. Immunol. 2018, 28, 197–199. [Google Scholar] [CrossRef]
- Meurs, H.; Gosens, R.; Zaagsma, J. Airway hyperresponsiveness in asthma: Lessons from in vitro model systems and animal models. Eur. Respir. J. 2008, 32, 487–502. [Google Scholar] [CrossRef]
- Sen, P.; Majumdar, U.; Zein, J. Inhaled corticosteroids do not adversely impact outcomes in COVID-19 positive patients with COPD: An analysis of Cleveland Clinic’s COVID-19 registry. PLoS ONE 2021, 16, e0252576. [Google Scholar] [CrossRef]
- Losappio, L.; Heffler, E.; Carpentiere, R. Characteristics of patients admitted to emergency department for asthma attack: A real-LIFE study. BMC Pulm. Med. 2019, 19, 107. [Google Scholar] [CrossRef]
- Nwaru, B.I.; Ekström, M.; Hasvold, P. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: A nationwide cohort study of the global SABINA programme. Eur. Respir. J. 2020, 55, 1901872. [Google Scholar] [CrossRef] [Green Version]
- Lacedonia, D.; Scioscia, G.; Santomasi, C. Impact of smoking, COPD and comorbidities on the mortality of COVID-19 patients. Sci. Rep. 2021, 11, 19251. [Google Scholar] [CrossRef]
- Meza, D.; Khuder, B.; Bailey, J.I. Mortality from COVID-19 in Patients with COPD: A US Study in the N3C Data Enclave. Int. J. Chron. Obstruct. Pulmon. Dis. 2021, 16, 2323–2326. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Athar, M. Mechanical ventilation in patients with chronic obstructive pulmonary disease and bronchial asthma. Indian J. Anaesth. 2015, 59, 589–598. [Google Scholar] [CrossRef]

| Patients with Asthma | Patients with COPD | Asthma vs. COPD | Patients with No Obstructive Lung Disease (Controls) | Asthma vs. Controls | |
|---|---|---|---|---|---|
| 39 | 71 | p | 906 | p | |
| Females, n (%) | 20 (51.3%) | 20 (28.2%) | 0.016 | 354 (39.1%) | n.s. |
| Age, years (mean [range]) | 59.2 [14–96] | 76.8 [42–94] | <0.001 | 64.7 [14–96] | 0.037 |
| Charlson Comorbidity Index (CCI) (mean ± SD) | 3.4 ± 1.9 | 6.2 ± 2.5 | <0.001 | 3.5 ± 2.6 | n.s. |
| Smoking habits | |||||
| Non-smokers, n (%) | 25 (64.1%) | 6 (8.5%) | <0.001 | 417 (46.0%) | n.s. |
| Smokers, n (%) | 3 (7.7%) | 13 (18.3%) | <0.001 | 157 (17.3%) | n.s. |
| Former smokers, n (%) | 6 (15.4%) | 50 (70.4%) | <0.001 | 282 (31.1%) | n.s. |
| Not declared, n (%) | 5 (12.8%) | 2 (2.8%) | <0.001 | 50 (5.6%) | n.s. |
| Variable | Patients with Asthma | Patients with COPD | Asthma vs. COPD | Patients with No Obstructive Lung Disease (Controls) | Asthma vs. Controls |
|---|---|---|---|---|---|
| Patient Number | 39 | 71 | p | 906 | p |
| Length of hospital stay, days (mean ± SD) | 17.4 ± 19.2 | 24.5 ± 23.7 | n.s. | 18.1 ± 15.8 | n.s. |
| Complications | |||||
| Respiratory failure, n (%) | 28 (71.8%) | 59 (83.1%) | n.s. | 641 (70.8%) | n.s. |
| COVID-19 pneumonia, n (%) | 21 (53.8%) | 47 (66.2%) | 0.022 | 622 (68.7%) | 0.005 |
| Ventilation | |||||
| No oxygen supports, n (%) | 5 (12.8%) | 4 (5.6%) | n.s. | 216 (23.8%) | n.s. |
| Nasal cannula (NC), n (%) | 8 (20.5%) | 14 (19.7%) | n.s. | 237 (26.2%) | n.s. |
| High-flow nasal oxygen (HFNO), n (%) | 13 (33.3.%) | 20 (28.2%) | n.s. | 130 (14.3%) | 0.001 |
| Non-invasive Ventilation (NIV), n (%) | 6 (15.4%) | 26 (36.6%) | 0.019 | 237 (26.2%) | n.s. |
| Invasive Mechanical Ventilation (IMV), n (%) | 7 (17.9%) | 7 (9.9%) | 0.044 | 86 (9.5%) | 0.048 |
| Hospital treatment | |||||
| Inhaled corticosteroids, n (%) | 12 (30.8%) | 18 (25.4%) | n.s. | 6 (0.7%) | <0.001 |
| Hydroxychloroquine, n (%) | 23 (59.0%) | 44 (62%) | n.s. | 657 (72.3%) | n.s. |
| Antiviral drugs, n (%) | 18 (46.2%) | 36 (50.7%) | n.s. | 541 (59.5%) | n.s. |
| Monoclonal antibodies, n (%) | 5 (12.8%) | 6 (8.5%) | n.s. | 161 (17.7%) | n.s. |
| Deaths, n (%) | 6 (15.4%) | 28 (39.4%) | <0.001 | 156 (17.2%) | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicola, S.; Borrelli, R.; Ridolfi, I.; Bernardi, V.; Borrelli, P.; Guida, G.; Antonelli, A.; Albera, C.; Marengo, S.; Briozzo, A.; et al. COPD, but Not Asthma, Is Associated with Worse Outcomes in COVID-19: Real-Life Data from Four Main Centers in Northwest Italy. J. Pers. Med. 2022, 12, 1184. https://doi.org/10.3390/jpm12071184
Nicola S, Borrelli R, Ridolfi I, Bernardi V, Borrelli P, Guida G, Antonelli A, Albera C, Marengo S, Briozzo A, et al. COPD, but Not Asthma, Is Associated with Worse Outcomes in COVID-19: Real-Life Data from Four Main Centers in Northwest Italy. Journal of Personalized Medicine. 2022; 12(7):1184. https://doi.org/10.3390/jpm12071184
Chicago/Turabian StyleNicola, Stefania, Richard Borrelli, Irene Ridolfi, Virginia Bernardi, Paolo Borrelli, Giuseppe Guida, Andrea Antonelli, Carlo Albera, Stefania Marengo, Antonio Briozzo, and et al. 2022. "COPD, but Not Asthma, Is Associated with Worse Outcomes in COVID-19: Real-Life Data from Four Main Centers in Northwest Italy" Journal of Personalized Medicine 12, no. 7: 1184. https://doi.org/10.3390/jpm12071184