1. Introduction
Atrial fibrillation (AF) is the prevailing cardiac arrhythmia in the western world. Prolonged lifespan and the connection with a plenty of other comorbidities contribute to the ever-growing AF incidence. Health and economic burden caused by AF alert the need for thorough investigation on its pathophysiology [
1]. AF springs principally from pulmonary veins (PVs) [
2] and propagates through cardiac structures [
3]. The main mechanism assisting the AF propagation is structural remodeling and fibrosis is especially contributing to the alteration of the cardiac anatomy, causing conduction heterogeneity, hence favoring the AF perpetuation [
1,
4]. Although conduction heterogeneity is more prominent during AF, the anatomical substrate can still be present for both atria even when patients are in sinus rhythm (SR) [
5,
6,
7]. As PVs are the main AF foci, their electrical isolation, called catheter ablation (CA), is the star AF treatment [
1,
8]. Despite the high CA success rates for paroxysmal AF patients, persistent AF cases often require the CA of additional cardiac structures that trigger or propagate the AF activity, known as non-PV triggers [
1,
3,
4,
9,
10,
11,
12].
Many techniques exist to localize non-PV triggers, with complex fractionated atrial electrograms (CFAEs) during AF [
4,
13,
14] or low voltage electrograms (EGMs) during SR [
4,
15,
16] being two of the most established ones. A combination of both techniques along with highly proportioned EGM fractionation has recently indicated sites showing fibrosis, with a high correlation between these areas in AF and SR [
6]. Nevertheless, the effect of CA on additional non-PV triggers remains quite controversial. Evidence shows that additional ablation of these sites offers little or no improved results with respect to single PVs ablation [
17,
18,
19]. It remains unclear, however, whether failure of additional CA applications to provide significant improvement in termination of AF stems from the incapacity of CA on sites other than PVs to terminate AF or from a vague and unclear definition of areas in need of ablation due to highly complex EGMs, thus highlighting the need for more reliable algorithms able to properly evaluate the atrial substrate [
6,
14].
So far, CA outcome on paroxysmal AF patients is primarily assessed from the analysis of the characteristics of P-waves, which represent the activation of the atria or heart-rate (HR) variability (HRV) analysis, which assesses the ventricular response, controlled by the autonomous nervous system (ANS). P-wave duration (PWD) is the most popular P-wave feature, reflecting the overall time that the wavefront needs to be propagated throughout the atria. Existence of prolonged or short PWD is considered an indicator of AF recurrence in paroxysmal or persistent AF patients, caused by conduction heterogeneity and scarring or shortening of the atrial refractory period, respectively [
20,
21,
22,
23,
24,
25]. PWD shortening is connected with the elimination of the conduction heterogeneity, hence being a favorable CA marker, while it is the second P-wave part, corresponding to left atrial depolarization, that is mainly modified after CA, possibly due to vicinity with PVs, the main object of CA [
26,
27,
28,
29]. PWD analysis goes beyond CA procedures, with application in studies predicting the AF occurrence or the risk for higher AF burden after pacemaker implantation [
25,
30,
31].
Apart from PWD, P-wave dispersion, amplitude, area or P-wave to R-peak interval are popular features utilized to predict AF recurrence [
26,
27,
32,
33]. P-wave analysis has been additionally applied to frequency domain in order to discern among healthy and AF subjects [
34]. HRV is a marker of fine tuning of ANS, which consists of sympathetic and parasympathetic systems and controls sinus rhythm. Evidence shows that people with low HRV are susceptible to AF [
35,
36,
37]. Energy delivered by radiofrequency (RF) CA (RFCA) can disturb the balance between sympathetic and parasympathetic systems, by stimulating the former and leading to temporary withdrawal of the latter, hence causing HRV attenuation, which in turn has been associated with AF recurrence [
38,
39,
40].
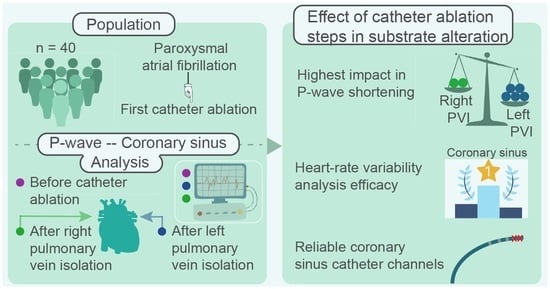
The number of studies and techniques aiming to analyze the CA effect on the atrial substrate is endless. At the same time, critical CA steps and their impact on the atrial substrate alteration is a rather neglected analysis field. Firstly, the aforementioned studies observing P-wave and HRV alterations only employ recordings acquired before and after CA. This postulates the theory of a uniform impact of left (LPVI) and right PVs isolation (RPVI). It should be considered, though, the possibility of each PV side playing a different role in atrial substrate alteration and hence, in AF activity, a conjecture that can be easily verified by employing signals recorded in between the ablation of LPVI and RPVI, which are already available in the recordings of any electrophysiology laboratory during stepwise CA procedures.
Coronary sinus’ (CS) strategical position between left (LA) and right atrium (RA) allows the detection of non-PV triggers and PV reconnection gaps throughout the atria during CA procedures via CS catheterization [
41,
42,
43,
44,
45,
46,
47,
48,
49]. Despite its extensive use as a CA reference, whether CS analysis could provide reliable information with respect to the AF substrate modification or which channels of CS catheter are the most appropriate for the analysis are two vital issues that remain unexplored. During CS cannulation, the most proximal pair of electrodes (9–10) is placed close to RA and the most distal pair (1–2) close to LA [
44,
47]. Notwithstanding, CS catheterization may be rather challenging due to variable CS anatomy and shape, aggrravated by myocardial contraction or the existence of CS dilation, factors that can lead to unstable recordings, especially from the distal tip of the catheter [
50,
51,
52,
53]. Additionally, anatomical alterations of the, adjacent to CS extremes, mitral annulus across the cardiac cycle in SR may affect furthermore the stability of recordings acquired from distal and proximal electrodes of the CS catheter [
54,
55]. Considering the aforementioned factors, information recorded across the CS catheter electrodes could vary significantly and the choice of the appropriate channel recruited for the analysis should be made with extreme caution.
The present work aims to elucidate the aforeposed issues regarding the CA procedure, in order to arise the understanding on the mechanisms of important CA steps and their interaction with the CA result. In the first place, the ability of CS channels to describe with the highest precision possible the AF dynamics during SR is assessed and the most and least recommended CS channels are defined. Afterwards, the relevance of CS in substrate modification evaluation due to CA is investigated via analysis of features traditionally applied to ECG recordings and cross-referenced by P-waves and HRV analysis of the ECGs. Finally, the evolution of P-waves and CS LAWs after isolation of either sides of PVs is tracked in order to define the PV side that has the highest impact in atrial substrate modification due to CA.
The manuscript is organized as follows.
Section 2 briefly describes the database recruited for the analysis, as well as the preprocessing and analysis steps.
Section 3 presents the results, which are further interpreted in
Section 4. Main findings are stated in
Section 5.
4. Discussion
This multi-approach study had three main objectives. First of all, to define the CS channels that can record with the highest precision and robustness the AF dynamics during SR. The analysis revealed the existence of variability among CS channels especially in Duration and Amplitude features. Differences were mostly found betweeen distal and medial and between distal and mid-proximal, with a trend between mid-distal and medial channels. A combined interpretation of the analysis of medians and the one-vs-all analysis indicates that distal channel showed the longest Duration, whilst the shortest Duration has been recorded by the medial channel. Regarding Amplitude and Area values, these were smaller in distal channel and larger in medial and mid-proximal channels. Proximal area showed the strongest morphological correlations between its channels. On the contrary, correlations in distal area or between distal and proximal channels were weaker. Hence, being the least susceptible channels to exogenous factors during SR, medial and mid-proximal channels are recommended while distal and mid-distal channels are not suggested.
Various studies corroborate these conclusions. CS EGM fractionation analysis was related with AF recurrence during SR in proximal and medial but not in distal channel in a recent study [
47]. Fractionation of proximal CS EGMs indicated AF patients in another study employing recordings during AF [
74]. Another work found that AF cycle length analysis in distal channel failed to predict AF termination after CA. In the same study, only mid-proximal channel predicted freedom from AF recurrence [
75].
The second objective of the present study was to investigate if CS recordings can describe adequately the substrate modification due to CA, as observed by P-wave analysis. Parallel P-waves and LAWs analysis has been conducted for this purpose. The former represent the entire atria while the latter provide very specific yet crucial information on CS function. Variations were observed to a higher degree in most of the features in P-waves than LAWs.
MV and ARV features were modified to an exceptionally higher extent in LAWs than P-waves. As CS recordings are closer to the tissue under ablation than surface ECG recordings, variability caused by RF energy deliverance may be illustrated with higher precision by LAWs [
38,
39]. Variation was more consistent in P-waves than LAWs, while the latter showed higher
Dispersion in values across all features.
The last but not least purpose of this work was the evaluation of additional recordings acquired during CA in order to understand the role that the ablation of each PV side plays to the modification of the studied features and, as a consequence, to the atrial substrate alteration. A significant P-wave shortening was observed after CA of both PV sides, in line with a plethora of previous studies mainly attributed to fibrotic areas causing conduction delays [
20,
21,
22,
23]. Interestingly enough, this reduction was observed right after LPVI, with RPVI not showing any additional effect to this feature.
Duration before CA was 120 ms, dropping down to 104 ms after LPVI and showing a minor increase after RPVI, to
ms. P-wave
Amplitude also tended to show a lower value after LPVI which was slightly increased after RPVI, but remained overall smaller than the pre-ablative measurements.
HRV attenuation after CA is considered an indicator of CA success [
38,
39,
40]. In line with previous studies, ARV in the present study showed a non-significant reduction after the end of the procedure. Nevertheless, recordings obtained after LPVI showed a trend for amplification of ARV values by up to
. Previous works studying the effect of RF energy in rabbits and students in lying position found that RF exposure can cause HRV incrementation and HR attenuation [
76,
77]. These findings explain the aforementioned results of the present study. Additionally, HR was indeed found to decrease after LPVI in the present work. Finally, HR-adjustment preserved the variation that was observed in
Duration after LPVI, although losing statistical power. It also incremented the different effect that RPVI had on
Duration, showing a slight non-statistical increase of
after RPVI. The reason for this slight incrementation is the HR acceleration after the end of CA procedure with respect to recordings after LPVI, where RF energy deliverance was still going on. Higher HR leads to generally narrower P-waves, the size of which is retrieved after HR-adjustment. An additional factor that may have a minor effect on this incrementation is a possible deviation of 1–2 ms in P-wave delineation precision, which due to its size (<+2.00% of duration values range) is considered acceptable.
Amplitude in P-waves showed a trend for reduction after LPVI. Although final
Amplitude was non-statistically reduced with respect to the beginning of the procedure, as in previous studies [
27], RPVI slightly but non-significantly increased
Amplitude values. Contrastly,
Slope rate was non-significantly increased after LPVI but decreased after RPVI in most of the studied time instances. It is highly possible that RF exposure also has had an effect on
Amplitude and
Slope rate features, explaining these variations.
Regarding LAWs, variations of most features show weaker statistical power and lower POV at each ablation step with respect to P-waves analysis. As mentioned afore,
MV and ARV varied more prominently in LAWs than P-waves.
MV showed a high incrementation in the order of
after LPVI and a decrease of
after RPVI. The reasons for these dramatic changes are not clear. Exposure to RF energy, not only affecting ARV but also
MV may be an explanation. Recently a study found
MV in lead V1 P-waves of paroxysmal AF undergoing CA of PVs to decrease after the procedure, as a sign of a successful ablation [
78]. Apart from the fact that these results come from P-waves analysis, which in our case show a slight attenuation overall,
MV in the present analysis was extracted by a template of the 20 most similar activations and not by considering all activations of one recording. ARV was also increased after LPVI in LAWs and to a higher extent than P-waves (up to
), possibly due to proximity to the tissue under ablation as explained previously.
To our knowledge, this is the first complete comparative study to perform simultaneous P-waves and LAWs analysis on recordings obtained not only before and after but also during CA of PVs. Recently, a relevant study calculated the organization of ECG recordings before, during and after CA of PVs and did not find any step to affect significantly the organization indices under calculation [
79]. As many differences exist with respect to our study, a comparison would not be straightforward. In the first place, individuals studied were persistent AF patients while the present study employed exclusively paroxysmal AF patients. As persistent AF shows more complicated atrial substrate and often presents AF drivers outside of PVs, efficiency of CA of PVs is notably lower with respect to success rates in paroxysmal AF patients [
19,
80]. Secondly, the procedure consisted of CA of PVs, CA of CFAEs and linear CA of LA. Recordings during the procedure were the recordings after CA of PVs and before CA of CFAEs and linear CA of LA. Hence, the intermediate stage of their analyis would be the final stage of ours and no information is provided about the contribution of left or right PVs. Finally, features employed are different from the features employed in the present study.
The key aspects of CA of PVs for paroxysmal AF patients investigated in the present study improve significantly the understanding of the AF mechanisms during SR and contribute to the knowledge on how these mechanisms respond to each step of CA. Moreover, the CA procedure itself is reconsidered and the most reliable means to analyze CS EGMs are explored. Overall, a more detailed perspective of the CA procedure and the effect of RF exposure to atrial tissue is obtained.
5. Conclusions
LPVI is the critical part of CA of PVs for paroxysmal AF patients, altering significantly the P-wave duration. RF exposure tends to cause temporary ARV incrementation, which is reversed right after the end of the CA procedure. The effect of CA of PVs on CS is less straightforward and takes place to a lesser extent. Thus, other atrial structures may be more indicative of the ablation outcome and should be assessed as alternative references.
It should be noted, however, that ARV modifications regarding RF energy are more prominently observed in CS LAWs, possibly due to the vicinity with the tissue under RF exposure. Hence, the employment of CS recordings may be beneficial for the study of ARV alterations during and after CA of PVs.
Finally, studies interested in employing CS analysis are encouraged to extract and investigate medial or mid-proximal channels, as they were found to be the most robust, showing the highest coherence between LAWs morphologies. Distal and mid-distal channels, on the other hand, should be avoided as they were prone to variable morphology and less clear activations.