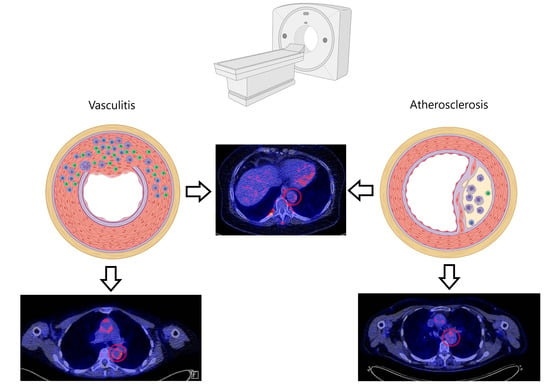
A Review on the Value of Imaging in Differentiating between Large Vessel Vasculitis and Atherosclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Questions
2.2. Search Strategy
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Extraction of Study Characteristics
3. Results
3.1. Ultrasound
3.2. FDG-PET
3.3. CT(A)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Search string MEDLINE (search date: 01-12-2020, 424 results)
- (“Diagnostic Imaging”[Mesh] OR Diagnostic Ima*[tiab] OR Ultraso*[tiab] OR US[tiab] OR magnetic resonance [tiab] OR MRI[tiab] OR MRA[tiab] OR angiography[tiab] OR computed tomography[tiab] OR CT[tiab] OR CTA[tiab] OR positron emission tomography[tiab] OR PET[tiab])
- AND
- (“Giant Cell Arteritis”[Mesh] OR Vasculi*[tiab] OR LVV[tiab] OR large vessel vasculitis[tiab] OR giant cell arteritis[tiab] OR GCA[tiab] OR “Takayasu Arteritis”[Mesh] OR takayasu arteritis[tiab] OR TAK[tiab])
- AND
- (“Atherosclerosis”[Mesh] OR “Arteriosclerosis”[Mesh] OR athero*[tiab] OR vascular calcification[tiab])
- NOT “Case Reports” [Publication Type]
- Search string EMBASE (search date: 01-12-2020, 519 results)
- ((‘Diagnostic Ima*’ OR Ultraso* OR US OR ‘magnetic resonance’ OR MRI OR MRA OR angiography OR ‘computed tomography’ OR CT OR CTA OR ‘positron emission tomography’ OR PET):ab,ti)
- AND
- ((Vasculi* OR LVV OR ‘large vessel vasculitis’ OR ‘giant cell arteritis’ OR GCA OR ‘takayasu arteritis’ OR TAK):ab,ti)
- AND
- ((athero* OR ‘vascular calcification’):ab,ti)
- NOT ‘case report’/exp
- Search string Web of Science, Core Collection (search date: 01-12-2020, 104 results)
- TS = (“Diagnostic Imaging” OR “Diagnostic Ima*’’ OR “Ultraso*’’ OR “US’’ OR “magnetic resonance’’ OR “MRI’’ OR “MRA’’ OR “angiography’’ OR “computed tomography’’ OR “CT’’ OR “CTA’’ OR “positron emission tomography’’ OR “PET’’)
- AND
- TI = (“Giant Cell Arteritis” OR ‘’Vasculi*’’ OR ‘’LVV’’ OR ‘’large vessel vasculitis’’ OR ‘’giant cell arteritis’’ OR ‘’GCA’’ OR “Takayasu Arteritis” OR ‘’TAK’’)
- AND
- TS = (“Atherosclerosis” OR “Arteriosclerosis” OR ‘’athero*’’ OR ‘’vascular calcification’’)
References
- Jennette, J. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin. Exp. Nephrol. 2013, 17, 603–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, R.; Berry, G.J.; Liang, D.H.; Goronzy, J.J.; Weyand, C.M. Pathogenesis of Giant Cell Arteritis and Takayasu Arteritis—Similarities and Differences. Curr. Rheumatol. Rep. 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Van Der Geest, K.S.M.; Sandovici, M.; Brouwer, E.; Mackie, S.L. Diagnostic Accuracy of Symptoms, Physical Signs, and Laboratory Tests for Giant Cell Arteritis: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2020, 180, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.H.; Beckman, J. Takayasu arteritis: Challenges in diagnosis and management. Heart 2018, 104, 558–565. [Google Scholar] [CrossRef]
- Luqmani, R.; Lee, E.; Singh, S.; Gillett, M.; Schmidt, W.A.; Bradburn, M.; Dasgupta, B.; Diamantopoulos, A.P.; Forrester-Barker, W.; Hamilton, W.; et al. The role of ultrasound compared to biopsy of temporal arteries in the diagnosis and treatment of giant cell arteritis (TABUL): A diagnostic accuracy and cost-effectiveness study. Health Technol Assess 2016, 20, 313. [Google Scholar] [CrossRef] [Green Version]
- MacKie, S.L.; Brouwer, E. What can negative temporal artery biopsies tell us? Rheumatology 2020, 59, 925–927. [Google Scholar] [CrossRef]
- Dejaco, C.; Ramiro, S.; Duftner, C.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Brouwer, E.; Cimmino, M.A.; Clark, E.; Dasgupta, B.; et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann. Rheum. Dis. 2018, 77, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Larivière, D.; Sacre, K.; Klein, I.; Hyafil, F.; Choudat, L.; Chauveheid, M.P.; Papo, T. Extra- and intracranial cerebral vasculitis in giant cell arteritis: An observational study. Medicine 2014, 93, e265. [Google Scholar] [CrossRef] [PubMed]
- Duftner, C.; Dejaco, C.; Sepriano, A.; Falzon, L.; Schmidt, W.A.; Ramiro, S. Imaging in diagnosis, outcome prediction and monitoring of large vessel vasculitis: A systematic literature review and meta-Analysis informing the EULAR recommendations. RMD Open 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- De Miguel, E.; Beltran, L.M.; Monjo, I.; Deodati, F.; Schmidt, W.A.; Garcia-Puig, J. Atherosclerosis as a potential pitfall in the diagnosis of giant cell arteritis. Rheumatology 2018, 57, 318–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Haim, S.; Kupzov, E.; Tamir, A.; Israel, O. Evaluation of18F-FDG uptake and arterial wall calcifications using18F-FDG PET/CT. J. Nucl. Med. 2004, 45, 1816–1821. [Google Scholar] [PubMed]
- Lensen, K.D.F.; Comans, E.F.I.; Voskuyl, A.E.; Van Der Laken, C.J.; Brouwer, E.; Zwijnenburg, A.T.; Pereira Arias-Bouda, L.M.; Glaudemans, A.W.J.M.; Slart, R.H.J.A.; Smulders, Y.M. Large-vessel vasculitis: Interobserver agreement and diagnostic accuracy of 18F-FDG-PET/CT. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Chareonthaitawee, P.; Treglia, G.; Besson, F.L.; Bley, T.A.; Blockmans, D.; Boellaard, R.; Bucerius, J.; Carril, J.M.; et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: Joint procedural recommendation of the EANM, SNMMI, and the PET Interest Group (PIG), and endorsed by the ASNC. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1250–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, F.; Strobl, F.F.; Cyran, C.C.; Helck, A.D.; Hartmann, M.; Schindler, A.; Nikolaou, K.; Reiser, M.F.; Saam, T. Reproducibility and differentiation of cervical arteriopathies using in vivo high-resolution black-blood MRI at 3 T. Neuroradiology 2016, 58, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Broncano, J.; Vargas, D.; Bhalla, S.; Cummings, K.W.; Raptis, C.A.; Luna, A. CT and MR imaging of cardiothoracic vasculitis. Radiographics 2018, 38, 997–1021. [Google Scholar] [CrossRef] [PubMed]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Das, M.; Braunschweig, T.; Mühlenbruch, G.; Mahnken, A.H.; Krings, T.; Langer, S.; Koeppel, T.; Jacobs, M.; Günther, R.W.; Mommertz, G. Carotid Plaque Analysis: Comparison of Dual-Source Computed Tomography (CT) Findings and Histopathological Correlation. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murgatroyd, H.; Nimmo, M.; Evans, A.; MacEwen, C. The use of ultrasound as an aid in the diagnosis of giant cell arteritis: A pilot study comparing histological features with ultrasound findings. EYE 2003, 17, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Fernández, E.; Monjo-Henry, I.; Bonilla, G.; Plasencia, C.; Miranda-Carús, M.-E.; Balsa, A.; De Miguel, E. False positives in the ultrasound diagnosis of giant cell arteritis: Some diseases can also show the halo sign. Rheumatology 2020. [Google Scholar] [CrossRef]
- Karahaliou, M.; Vaiopoulos, G.; Papaspyrou, S.; Kanakis, M.A.; Revenas, K.; Sfikakis, P.P. Colour duplex sonography of temporal arteries before decision for biopsy: A prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res. Ther. 2006, 8, R116. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.-F.; Jeng, J.-S.; Lu, C.-J.; Yip, P.-K. Clinical and ultrasonographic manifestations in major causes of common carotid artery occlusion. J. Neuroimaging 2005, 15, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Grayson, P.C.; Alehashemi, S.; Bagheri, A.A.; Civelek, A.C.; Cupps, T.R.; Kaplan, M.J.; Malayeri, A.A.; Merkel, P.A.; Novakovich, E.; Bluemke, D.A.; et al. 18F-Fluorodeoxyglucose–Positron Emission Tomography As an Imaging Biomarker in a Prospective, Longitudinal Cohort of Patients With Large Vessel Vasculitis. Arthritis Rheumatol. 2018, 70, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stellingwerff, M.D.; Brouwer, E.; Lensen, K.J.D.F.; Rutgers, A.; Arends, S.; Van Der Geest, K.S.M.; Glaudemans, A.W.J.M.; Slart, R.H.J.A. Different scoring methods of FDG PET/CT in Giant cell arteritis need for standardization. Medicine 2015, 94, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, S.; Taneja, K.; Gupta, A.K.; Rajani, M. Morphologic mural changes in the aorta revealed by CT in patients with nonspecific aortoarteritis (Takayasu’s arteritis). Am. J. Roentgenol. 1996, 167, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, V.R.; Crowson, C.S.; Bhagra, A.S.; Warrington, K.J.; Vrtiska, T.J. CT angiographic imaging characteristics of thoracic idiopathic aortitis. J. Cardiovasc. Comput. Tomogr. 2013, 7, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Chrysidis, S.; Duftner, C.; Dejaco, C.; Schaefer, V.S.; Ramiro, S.; Carrara, G.; Scire, C.A.; Hocevar, A.; Diamantopoulos, A.P.; Iagnocco, A.; et al. Definitions and reliability assessment of elementary ultrasound lesions in giant cell arteritis: A study from the OMERACT Large Vessel Vasculitis Ultrasound Working Group. RMD Open 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Tarkin, J.M.; Joshi, F.R.; Rudd, J.H.F. PET imaging of inflammation in atherosclerosis. Nat. Rev. Cardiol. 2014, 11, 443–457. [Google Scholar] [CrossRef]
- Meirelles, G.S.P.; Gonen, M.; Strauss, H.W. 18F-FDG uptake and calcifications in the thoracic aorta on positron emission tomography/computed tomography examinations: Frequency and stability on serial scans. J. Thorac. Imaging 2011, 26, 54–62. [Google Scholar] [CrossRef]
- Slart, R.H.; Glaudemans, A.W.; Gheysens, O.; Lubberink, M.; Kero, T.; Dweck, M.R.; Habib, G.; Gaemperli, O.; Saraste, A.; Gimelli, A.; et al. Procedural recommendations of cardiac PET/CT imaging: Standardization in inflammatory-, infective-, infiltrative-, and innervation (4Is)-related cardiovascular diseases: A joint collaboration of the EACVI and the EANM. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Belhocine, T.; Blockmans, D.; Hustinx, R.; Vandevivere, J.; Mortelmans, L. Imaging of large vessel vasculitis with 18FDG PET: Illusion or reality? A critical review of the literature data. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1305–1313. [Google Scholar] [CrossRef]
- Bucerius, J.; Hyafil, F.; Verberne, H.J.; Slart, R.H.J.A.; Lindner, O.; Sciagra, R.; Agostini, D.; Übleis, C.; Gimelli, A.; Hacker, M. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Schlett, C.L.; Ferencik, M.; Celeng, C.; Maurovich-Horvat, P.; Scheffel, H.; Stolzmann, P.; Do, S.; Kauczor, H.U.; Alkadhi, H.; Bamberg, F.; et al. How to assess non-calcified plaque in CT angiography: Delineation methods affect diagnostic accuracy of low-attenuation plaque by CT for lipid-core plaque in histology. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto-González, S.; Arguis, P.; García-Martínez, A.; Espígol-Frigolé, G.; Tavera-Bahillo, I.; Butjosa, M.; Sánchez, M.; Hernández-Rodríguez, J.; Grau, J.M.; Cid, M.C. Large vessel involvement in biopsy-proven giant cell arteritis: Prospective study in 40 newly diagnosed patients using CT angiography. Ann. Rheum. Dis. 2012, 71, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Menezes, L.J.; Kotze, C.W.; Agu, O.; Richards, T.; Brookes, J.; Goh, V.J.; Rodriguez-Justo, M.; Endozo, R.; Harvey, R.; Yusuf, S.W.; et al. Investigating vulnerable atheroma using combined 18F-FDG PET/CT angiography of carotid plaque with immunohistochemical validation. J. Nucl. Med. 2011, 52, 1698–1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perren, F.; Vargas, M.I.; Kargiotis, O. Etiology of Intracranial Arterial Stenosis: Are Transcranial Color-Coded Duplex Ultrasound and 3T Black Blood MR Imaging Complementary? J. Neuroimaging 2016, 26, 426–430. [Google Scholar] [CrossRef]
- Park, J.E.; Jung, S.C.; Lee, S.H.; Jeon, J.Y.; Lee, J.Y.; Kim, H.S.; Choi, C.-G.; Kim, S.-O.S.J.; Lee, D.H.; Kim, S.-O.S.J.; et al. Comparison of 3D magnetic resonance imaging and digital subtraction angiography for intracranial artery stenosis. Eur. Radiol. 2017, 27, 4737–4746. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Maron, D.J.; Shirai, T.; Weyand, C.M. Accelerated atherosclerosis in patients with chronic inflammatory rheumatologic conditions. Int. J. Clin. Rheumtol. 2015, 10, 365–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rominger, A.; Saam, T.; Wolpers, S.; Cyran, C.C.; Schmidt, M.; Foerster, S.; Nikolaou, K.; Reiser, M.F.; Bartenstein, P.; Hacker, M. 18F-FDG PET/CT identifies patients at risk for future vascular events in an otherwise asymptomatic cohort with neoplastic disease. J. Nucl. Med. 2009, 50, 1611–1620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al’Aref, S.J.; Anchouche, K.; Singh, G.; Slomka, P.J.; Kolli, K.K.; Kumar, A.; Pandey, M.; Maliakal, G.; Van Rosendael, A.R.; Beecy, A.N.; et al. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur. Heart J. 2019, 40, 1975–1986. [Google Scholar] [CrossRef]

| First Author | Year | Imaging Modality | Primary Aim | Primary Outcome |
|---|---|---|---|---|
| Sharma | 1995 | CT | Assess vessel wall changes in TA | TA patients show distinct changes in vessel wall morphology |
| Murgatroyd | 2003 | US | Evaluate the diagnostic accuracy of US in GCA | US shows a sensitivity 86% and a specificity of 68% |
| Tsai | 2005 | US | Identify the main cause of carotid artery occlusion | Atherosclerosis and TA are the two most common causes of carotid artery occlusion |
| Karahaliou | 2006 | US | Evaluate the diagnostic accuracy of US in GCA | US shows high sensitivity when bilateral halo sign is present |
| Chowdhary | 2013 | CT | Identify CT angiographic findings in aortitis | Idiopathic aortitis causes larger dilatation than noninflammatory aneurysms |
| Stellingwerff | 2015 | FDG-PET | To define optimal scoring methods for GCA | Visual scoring of vascular uptake compared to liver demonstrated the highest accuracy |
| Grayson | 2018 | FDG-PET | Assessing the role of FDG-PET as a biomarker in GCA | Higher FDG-PET scores resulted in a higher chance of relapse |
| Fernàndez-Fernàndez | 2020 | US | Frequency of US halo sign in non-GCA patients | There are other conditions than GCA that reveal the halo sign |
| First Author | Year | Study Design | Vasculitis Patients | Atherosclerosis Patients | Presence of Hypoechoic Ring (Halo Sign) Temporal Artery (%) | Blood Flow Abnormality (%) | Homogenous Echogenicity Carotid Artery (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Vasculitis (GCA; TA) | Reference Diagnosis | Number of Patients | Mean Age | Reference Diagnosis | Number of Patients | Mean Age | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | |||
| Murgatroyd | 2003 | Prospective | GCA | Positive Temporal Artery Biopsy | 7 | - | Histology | 8 | - | 6 (86) | 6 (75) | - | - | - | - |
| Tsai | 2005 | Prospective | TA | Ishikawa Criteria | 11 | 36 | Clinical Diagnosis | 17 | 70 | - | - | - | - | 0 (0) | 11 (100) |
| Karahaliou | 2006 | Prospective | GCA | Clinical Diagnosis | 22 | 70 | Clinical Diagnosis of DM Type II or Stroke | 15 | 73 | 18 (82) | 0 (0) | 9 (41) | 6 (40) | - | - |
| Fernàndez-Fernàndez | 2020 | Retrospective | GCA | Clinical Diagnosis | 291 | - | 3 | - | 291 * (100) | 3 * (100) | - | - | - | - | |
| First Author | Year | Study Design | Vasculitis Patients | Atherosclerosis Patients | Number of Patients with Visual Uptake Similar to Liver (%); Higher than Liver (%) | Number of Patients with Diffuse Visual Uptake | Mean Number of Arteries with Increased Visual FDG Uptake (range) | Mean SUVmax in the Aorta (SD) | Number of Scans ** with Positive Visual ‘Gestalt’ LVV Assessment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Vasculitis (GCA; TA) | Reference Diagnosis | Number of Patients | Mean Age | Reference Diagnosis | Number of Patients | Mean Age | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | |||
| Stellingwerff | 2015 | Retrospective | GCA | ACR Criteria; Positive TAB; Established Clinical Diagnosis | 12 | 70 | CT Calcified Plaque Score > 2 | 19 | 69 | 12 (100); 11 (92) | 12 (63); 4 (21) | 12 (100) | 4 (21) | 35 (19-40) | 13 (5-27) | 3.83 (1.10) | 2.82 (0.76) | - | - |
| Grayson | 2018 | Prospective | GCA; TA * | ACR Criteria; Clinically Active Disease | 25; 15 * | 67; 44 * | Hyperlipidemia (>55 years and statin use) | 35 | 64 | - | - | - | - | 22 (-); 19 * (-) *** | 14 *** | - | - | 34 (85) | 6 (17) |
| First Author | Year | Study Design | Vasculitis Patients | Atherosclerosis Patients | Patients with Aortic Stenosis or occlusion (%) | Patients with Aortic Dilative Lesions (%) | Patients with Aortic Wall Thickening (%) | Patients with Aortic Calcification (%) | Diameter Ascending Aorta mm (SD) | Diameter Aortic Arch mm (SD) | Diameter Descending Aorta mm (SD) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Vasculitis (GCA; TA) | Reference Diagnosis | Number of Patients | Mean Age | Reference Diagnosis | Number of Patients | Mean Age | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | Vasculitis Patients | Atherosclerosis Patients | |||
| Sharma | 1996 | Prospective | TA | - | 24 | 70 | - | 12 | 63 | 10 (42) | 0 (0) | 9 (38) | 0 (0) | 20 (83) | 0 (0) | 13 (54) | 12 (100) | - | - | - | - | - | - |
| Chowdhary | 2013 | Retrospective | GCA **** | Clinical Diagnosis of Secondary Aortitis | 16 | 36 | Patients with noninflammatory aneurysms | 18 | 70 | - | - | - | - | 1 (6) | 4 (22) | 1 (6) | 10 (56) | 53 (10) | 49 (12) | 35 (6) | 31 (4) | 36 (7) | 33 (13) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nienhuis, P.H.; van Praagh, G.D.; Glaudemans, A.W.J.M.; Brouwer, E.; Slart, R.H.J.A. A Review on the Value of Imaging in Differentiating between Large Vessel Vasculitis and Atherosclerosis. J. Pers. Med. 2021, 11, 236. https://doi.org/10.3390/jpm11030236
Nienhuis PH, van Praagh GD, Glaudemans AWJM, Brouwer E, Slart RHJA. A Review on the Value of Imaging in Differentiating between Large Vessel Vasculitis and Atherosclerosis. Journal of Personalized Medicine. 2021; 11(3):236. https://doi.org/10.3390/jpm11030236
Chicago/Turabian StyleNienhuis, Pieter H., Gijs D. van Praagh, Andor W. J. M. Glaudemans, Elisabeth Brouwer, and Riemer H. J. A. Slart. 2021. "A Review on the Value of Imaging in Differentiating between Large Vessel Vasculitis and Atherosclerosis" Journal of Personalized Medicine 11, no. 3: 236. https://doi.org/10.3390/jpm11030236