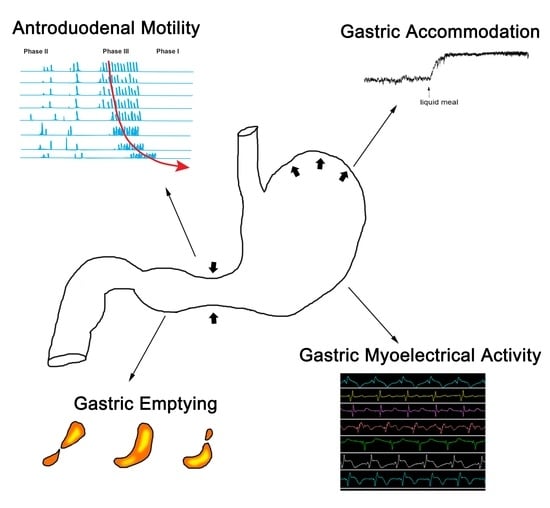
Diagnostic Methods for Evaluation of Gastric Motility—A Mini Review
Abstract
:1. Introduction
2. Assessment of Gastric Accommodation
2.1. Barostat
2.2. Satiation Drinking Test
2.3. Single Photon Emission Computed Tomography
2.4. Ultrasonography
3. Assessment of Antroduodenal Motility
3.1. Antroduodenal Manometry
3.2. Magnetic Resonance Imaging
4. Assessment of Gastric Emptying
4.1. Gastric Emptying Scintigraphy
4.2. Gastric Emptying Breath Test
4.3. Wireless Motility Capsule
5. Assessment of Gastric Myoelectrical Activity
5.1. Electrogastrography
5.2. High-Resolution Electrogastrography
5.3. Body Surface Gastric Mapping
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, A.C.; Mahadeva, S.; Carbone, M.F.; Lacy, B.E.; Talley, N.J. Functional dyspepsia. Lancet 2020, 396, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Stanghellini, V.; Chan, F.K.; Hasler, W.L.; Malagelada, J.R.; Suzuki, H.; Tack, J.; Talley, N.J. Gastroduodenal Disorders. Gastroenterology 2016, 150, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Kuo, B.; Nguyen, L.; Vaughn, V.M.; Petrey, J.; Greer, K.; Yadlapati, R.; Abell, T.L. ACG Clinical Guideline: Gastroparesis. Am. J. Gastroenterol. 2022, 117, 1197–1220. [Google Scholar] [CrossRef]
- Camilleri, M.; Sanders, K.M. Gastroparesis. Gastroenterology 2022, 162, 68–87.e61. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Farrugia, G.; Stanghellini, V. Gastroparesis: A turning point in understanding and treatment. Gut 2019, 68, 2238–2250. [Google Scholar] [CrossRef]
- Wadhwa, V.; Mehta, D.; Jobanputra, Y.; Lopez, R.; Thota, P.N.; Sanaka, M.R. Healthcare utilization and costs associated with gastroparesis. World J. Gastroenterol. 2017, 23, 4428–4436. [Google Scholar] [CrossRef]
- Schol, J.; Wauters, L.; Dickman, R.; Drug, V.; Mulak, A.; Serra, J.; Enck, P.; Tack, J. United European Gastroenterology (UEG) and European Society for Neurogastroenterology and Motility (ESNM) consensus on gastroparesis. United Eur. Gastroenterol. J. 2021, 9, 287–306. [Google Scholar] [CrossRef]
- Kim, B.J.; Kuo, B. Gastroparesis and Functional Dyspepsia: A Blurring Distinction of Pathophysiology and Treatment. J. Neurogastroenterol. Motil. 2019, 25, 27–35. [Google Scholar] [CrossRef]
- Salet, G.A.; Samsom, M.; Roelofs, J.M.; van Berge Henegouwen, G.P.; Smout, A.J.; Akkermans, L.M. Responses to gastric distension in functional dyspepsia. Gut 1998, 42, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Tack, J.; Piessevaux, H.; Coulie, B.; Caenepeel, P.; Janssens, J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology 1998, 115, 1346–1352. [Google Scholar] [CrossRef]
- Karamanolis, G.; Caenepeel, P.; Arts, J.; Tack, J. Determinants of symptom pattern in idiopathic severely delayed gastric emptying: Gastric emptying rate or proximal stomach dysfunction? Gut 2007, 56, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.J.; Kindt, S.; Tack, J. Pathophysiology of functional dyspepsia. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Bjornsson, E.S.; Abrahamsson, H. Contractile patterns in patients with severe chronic dyspepsia. Am. J. Gastroenterol. 1999, 94, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, G. Interstitial cells of Cajal in health and disease. Neurogastroenterol. Motil. 2008, 20 (Suppl. S1), 54–63. [Google Scholar] [CrossRef]
- Lin, Z.; Sarosiek, I.; Forster, J.; Damjanov, I.; Hou, Q.; McCallum, R.W. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol. Motil. 2010, 22, 56–61.e10. [Google Scholar] [CrossRef]
- Bekkelund, M.; Sangnes, D.A.; Gunnar Hatlebakk, J.; Aabakken, L. Pathophysiology of idiopathic gastroparesis and implications for therapy. Scand. J. Gastroenterol. 2019, 54, 8–17. [Google Scholar] [CrossRef]
- Carbone, F.; De Buysscher, R.; Van den Houte, K.; Schol, J.; Goelen, N.; Tack, J. Relationship Between Gastric Emptying Rate and Simultaneously Assessed Symptoms in Functional Dyspepsia. Clin. Gastroenterol. Hepatol. 2022, 20, e429–e437. [Google Scholar] [CrossRef]
- Cangemi, D.J.; Lacy, B.E. Gastroparesis. Curr. Opin. Gastroenterol. 2021, 37, 596–601. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Camilleri, M.; Chedid, V.; Mandawat, A.; Erwin, P.J.; Murad, M.H. Effects of Promotility Agents on Gastric Emptying and Symptoms: A Systematic Review and Meta-analysis. Gastroenterology 2019, 156, 1650–1660. [Google Scholar] [CrossRef]
- Lin, Z.; Eaker, E.Y.; Sarosiek, I.; McCallum, R.W. Gastric myoelectrical activity and gastric emptying in patients with functional dyspepsia. Am. J. Gastroenterol. 1999, 94, 2384–2389. [Google Scholar] [CrossRef]
- Chen, J.; McCallum, R.W. Gastric slow wave abnormalities in patients with gastroparesis. Am. J. Gastroenterol. 1992, 87, 477–482. [Google Scholar]
- Koch, K.L. Gastric dysrhythmias: A potential objective measure of nausea. Exp. Brain Res. 2014, 232, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Gharibans, A.A.; Calder, S.; Varghese, C.; Waite, S.; Schamberg, G.; Daker, C.; Du, P.; Alighaleh, S.; Carson, D.; Woodhead, J.; et al. Gastric dysfunction in patients with chronic nausea and vomiting syndromes defined by a noninvasive gastric mapping device. Sci. Transl. Med. 2022, 14, eabq3544. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, R.D.; Alavi, A.; Reynolds, J.C. Electrogastrography in patients with gastroparesis and effect of long-term cisapride. Dig. Dis. Sci. 1993, 38, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Forster, J.; Damjanov, I.; Lin, Z.; Sarosiek, I.; Wetzel, P.; McCallum, R.W. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J. Gastrointest. Surg. 2005, 9, 102–108. [Google Scholar] [CrossRef]
- O’Grady, G.; Angeli, T.R.; Du, P.; Lahr, C.; Lammers, W.; Windsor, J.A.; Abell, T.L.; Farrugia, G.; Pullan, A.J.; Cheng, L.K. Abnormal initiation and conduction of slow-wave activity in gastroparesis, defined by high-resolution electrical mapping. Gastroenterology 2012, 143, 589–598.e583. [Google Scholar] [CrossRef] [Green Version]
- Angeli, T.R.; Cheng, L.K.; Du, P.; Wang, T.H.; Bernard, C.E.; Vannucchi, M.G.; Faussone-Pellegrini, M.S.; Lahr, C.; Vather, R.; Windsor, J.A.; et al. Loss of Interstitial Cells of Cajal and Patterns of Gastric Dysrhythmia in Patients With Chronic Unexplained Nausea and Vomiting. Gastroenterology 2015, 149, 56–66.e55. [Google Scholar] [CrossRef] [Green Version]
- De Schepper, H.U.; Cremonini, F.; Chitkara, D.; Camilleri, M. Assessment of gastric accommodation: Overview and evaluation of current methods. Neurogastroenterol. Motil. 2004, 16, 275–285. [Google Scholar] [CrossRef]
- Thumshirn, M.; Camilleri, M.; Saslow, S.B.; Williams, D.E.; Burton, D.D.; Hanson, R.B. Gastric accommodation in non-ulcer dyspepsia and the roles of Helicobacter pylori infection and vagal function. Gut 1999, 44, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Tack, J.; Janssen, P.; Masaoka, T.; Farre, R.; Van Oudenhove, L. Efficacy of Buspirone, a Fundus-Relaxing Drug, in Patients with Functional Dyspepsia. Clin. Gastroenterol. Hepatol. 2012, 10, 1239–1245. [Google Scholar] [CrossRef] [Green Version]
- Febo-Rodriguez, L.; Chumpitazi, B.P.; Sher, A.C.; Shulman, R.J. Gastric accommodation: Physiology, diagnostic modalities, clinical relevance, and therapies. Neurogastroenterol. Motil. 2021, 33, e14213. [Google Scholar] [CrossRef] [PubMed]
- de Zwart, I.M.; Haans, J.J.; Verbeek, P.; Eilers, P.H.; de Roos, A.; Masclee, A.A. Gastric accommodation and motility are influenced by the barostat device: Assessment with magnetic resonance imaging. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G208–G214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, C.A.; Aghababaie, Z.; Paskaranandavadivel, N.; Avci, R.; Cheng, L.K.; Angeli-Gordon, T.R. Localized gastric distension disrupts slow-wave entrainment leading to temporary ectopic propagation: A high-resolution electrical mapping study. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G656–G667. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.L.; Hong, S.P.; Xu, L. Reproducibility of gastric myoelectrical activity and the water load test in patients with dysmotility-like dyspepsia symptoms and in control subjects. J. Clin. Gastroenterol. 2000, 31, 125–129. [Google Scholar] [CrossRef]
- Kindt, S.; Tack, J. Impaired gastric accommodation and its role in dyspepsia. Gut 2006, 55, 1685–1691. [Google Scholar] [CrossRef]
- Parkman, H.P.; Hallinan, E.K.; Hasler, W.L.; Farrugia, G.; Koch, K.L.; Nguyen, L.; Snape, W.J.; Abell, T.L.; McCallum, R.W.; Sarosiek, I.; et al. Early satiety and postprandial fullness in gastroparesis correlate with gastroparesis severity, gastric emptying, and water load testing. Neurogastroenterol. Motil. 2017, 29, e12981. [Google Scholar] [CrossRef] [Green Version]
- Scarpellini, E.; Van den Houte, K.; Schol, J.; Huang, I.H.; Colomier, E.; Carbone, F.; Tack, J. Nutrient Drinking Test as Biomarker in Functional Dyspepsia. Am. J. Gastroenterol. 2021, 116, 1387–1395. [Google Scholar] [CrossRef]
- Tack, J.; Caenepeel, P.; Piessevaux, H.; Cuomo, R.; Janssens, J. Assessment of meal induced gastric accommodation by a satiety drinking test in health and in severe functional dyspepsia. Gut 2003, 52, 1271–1277. [Google Scholar] [CrossRef] [Green Version]
- Tack, J.F.; Talley, N.J.; Kowalski, D.L.; Borton, M.A.; Barve, A. Influence of PPI run-in, pH monitoring and nutrient tolerance on efficacy outcomes of acotiamide hydrochloride (YM443), a novel acetylcholine esterase inhibitor, in functional dyspepsia. Gastroenterology 2008, 134, A143. [Google Scholar] [CrossRef]
- Dukes, G.E.; Scimia, C.; Kuo, B.; Zhang, W.; Gupta, S.; Chen, C.; Chuang, E.; Camilleri, M. Safety, tolerability and pharmacodynamics of TAK-906, a dopamine 2,3 antagonist, in patients with diabetic or idiopathic gastroparesis. Neurogastroenterol. Motil. 2019, 31, 1. [Google Scholar]
- Gonenne, J.; Castillo, E.J.; Camilleri, M.; Burton, D.; Thomforde, G.M.; Baxter, K.L.; Zinsmeister, A.R. Does the nutrient drink test accurately predict postprandial gastric volume in health and community dyspepsia? Neurogastroenterol. Motil. 2005, 17, 44–50. [Google Scholar] [CrossRef] [PubMed]
- van den Elzen, B.D.; Bennink, R.J.; Wieringa, R.E.; Tytgat, G.N.; Boeckxstaens, G.E. Fundic accommodation assessed by SPECT scanning: Comparison with the gastric barostat. Gut 2003, 52, 1548–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouras, E.P.; Delgado-Aros, S.; Camilleri, M.; Castillo, E.J.; Burton, D.D.; Thomforde, G.M.; Chial, H.J. SPECT imaging of the stomach: Comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Gut 2002, 51, 781–786. [Google Scholar] [CrossRef] [Green Version]
- Gilja, O.H.; Lunding, J.; Hausken, T.; Gregersen, H. Gastric accommodation assessed by ultrasonography. World J. Gastroenterol. 2006, 12, 2825–2829. [Google Scholar] [CrossRef]
- Bisschops, R.; Tack, J. Dysaccommodation of the stomach: Therapeutic nirvana? Neurogastroenterol. Motil. 2007, 19, 85–93. [Google Scholar] [CrossRef]
- Hausken, T.; Odegaard, S.; Matre, K.; Berstad, A. Antroduodenal motility and movements of luminal contents studied by duplex sonography. Gastroenterology 1992, 102, 1583–1590. [Google Scholar] [CrossRef]
- Manini, M.L.; Burton, D.D.; Meixner, D.D.; Eckert, D.J.; Callstrom, M.; Schmit, G.; El-Youssef, M.; Camilleri, M. Feasibility and application of 3-dimensional ultrasound for measurement of gastric volumes in healthy adults and adolescents. J. Pediatr. Gastroenterol. Nutr. 2009, 48, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Buisman, W.J.; van Herwaarden-Lindeboom, M.Y.; Mauritz, F.A.; El Ouamari, M.; Hausken, T.; Olafsdottir, E.J.; van der Zee, D.C.; Gilja, O.H. Validation of a Novel 3-Dimensional Sonographic Method for Assessing Gastric Accommodation in Healthy Adults. J. Ultrasound Med. 2016, 35, 1411–1418. [Google Scholar] [CrossRef] [Green Version]
- Steinsvik, E.K.; Hausken, T.; Gilja, O.H. The ultrasound meal accommodation test in 509 patients with functional gastrointestinal disorders. Scand. J. Gastroenterol. 2016, 51, 788–794. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Gonlachanvit, S. Technique of functional and motility test: How to perform antroduodenal manometry. J. Neurogastroenterol. Motil. 2013, 19, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storlid, E.L.; Hausken, T.; Lied, G.A.; Gilja, O.H.; Hatlebakk, J.G. Gastric accommodation in healthy subjects studied by ultrasound, manometry, and impedancemetry. Neurogastroenterol. Motil. 2018, 30, e13249. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Bassotti, G.; Clarke, J.; Dinning, P.; Fox, M.; Grover, M.; Hellström, P.M.; Ke, M.; Layer, P.; Malagelada, C.; et al. Expert consensus document: Advances in the diagnosis and classification of gastric and intestinal motility disorders. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 291–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Bharucha, A.E.; di Lorenzo, C.; Hasler, W.L.; Prather, C.M.; Rao, S.S.; Wald, A. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol. Motil. 2008, 20, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Brown, M.L.; Malagelada, J.R. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology 1986, 91, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Cogliandro, R.F.; Rizzoli, G.; Bellacosa, L.; De Giorgio, R.; Cremon, C.; Barbara, G.; Stanghellini, V. Is gastroparesis a gastric disease? Neurogastroenterol. Motil. 2019, 31, e13562. [Google Scholar] [CrossRef] [PubMed]
- Katzka, D.A.; Kahrilas, P.J. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ 2020, 371, m3786. [Google Scholar] [CrossRef] [PubMed]
- Hereijgers, M.J.M.; Keszthelyi, D.; Kruimel, J.W.; Masclee, A.A.M.; Conchillo, J.M. Antroduodenal motility recording identifies characteristic patterns in gastroparesis related to underlying etiology. Neurogastroenterol. Motil. 2022, 34, e14394. [Google Scholar] [CrossRef]
- Tack, J.; Deloose, E.; Ang, D.; Scarpellini, E.; Vanuytsel, T.; Van Oudenhove, L.; Depoortere, I. Motilin-induced gastric contractions signal hunger in man. Gut 2016, 65, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Goetze, O.; Steingoetter, A.; Menne, D.; van der Voort, I.R.; Kwiatek, M.A.; Boesiger, P.; Weishaupt, D.; Thumshirn, M.; Fried, M.; Schwizer, W. The effect of macronutrients on gastric volume responses and gastric emptying in humans: A magnetic resonance imaging study. Am. J. Physiol.-Gastrointest. Liver Physiol. 2007, 292, G11–G17. [Google Scholar] [CrossRef] [Green Version]
- Feinle, C.; Kunz, P.; Boesiger, P.; Fried, M.; Schwizer, W. Scintigraphic validation of a magnetic resonance imaging method to study gastric emptying of a solid meal in humans. Gut 1999, 44, 106–111. [Google Scholar] [CrossRef]
- Lu, K.H.; Liu, Z.; Jaffey, D.; Wo, J.M.; Mosier, K.M.; Cao, J.; Wang, X.; Powley, T.L. Automatic assessment of human gastric motility and emptying from dynamic 3D magnetic resonance imaging. Neurogastroenterol. Motil. 2022, 34, e14239. [Google Scholar] [CrossRef] [PubMed]
- Abell, T.L.; Camilleri, M.; Donohoe, K.; Hasler, W.L.; Lin, H.C.; Maurer, A.H.; McCallum, R.W.; Nowak, T.; Nusynowitz, M.L.; Parkman, H.P.; et al. Consensus recommendations for gastric emptying scintigraphy: A joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am. J. Gastroenterol. 2008, 103, 753–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tougas, G.; Eaker, E.Y.; Abell, T.L.; Abrahamsson, H.; Boivin, M.; Chen, J.; Hocking, M.P.; Quigley, E.M.; Koch, K.L.; Tokayer, A.Z.; et al. Assessment of gastric emptying using a low fat meal: Establishment of international control values. Am. J. Gastroenterol. 2000, 95, 1456–1462. [Google Scholar] [CrossRef]
- Desai, A.; O’Connor, M.; Neja, B.; Delaney, K.; Camilleri, M.; Zinsmeister, A.R.; Bharucha, A.E. Reproducibility of gastric emptying assessed with scintigraphy in patients with upper GI symptoms. Neurogastroenterol. Motil. 2018, 30, e13365. [Google Scholar] [CrossRef]
- Vijayvargiya, P.; Jameie-Oskooei, S.; Camilleri, M.; Chedid, V.; Erwin, P.J.; Murad, M.H. Association between delayed gastric emptying and upper gastrointestinal symptoms: A systematic review and meta-analysis. Gut 2019, 68, 804–813. [Google Scholar] [CrossRef]
- Orthey, P.; Yu, D.; Van Natta, M.L.; Ramsey, F.V.; Diaz, J.R.; Bennett, P.A.; Iagaru, A.H.; Fragomeni, R.S.; McCallum, R.W.; Sarosiek, I.; et al. Intragastric Meal Distribution During Gastric Emptying Scintigraphy for Assessment of Fundic Accommodation: Correlation with Symptoms of Gastroparesis. J. Nucl. Med. 2018, 59, 691–697. [Google Scholar] [CrossRef]
- Chedid, V.; Halawi, H.; Brandler, J.; Burton, D.; Camilleri, M. Gastric accommodation measurements by single photon emission computed tomography and two-dimensional scintigraphy in diabetic patients with upper gastrointestinal symptoms. Neurogastroenterol. Motil. 2019, 31, e13581. [Google Scholar] [CrossRef]
- Pasricha, P.J.; Grover, M.; Yates, K.P.; Abell, T.L.; Bernard, C.E.; Koch, K.L.; McCallum, R.W.; Sarosiek, I.; Kuo, B.; Bulat, R.; et al. Functional Dyspepsia and Gastroparesis in Tertiary Care are Interchangeable Syndromes With Common Clinical and Pathologic Features. Gastroenterology 2021, 160, 2006–2017. [Google Scholar] [CrossRef]
- Szarka, L.A.; Camilleri, M.; Vella, A.; Burton, D.; Baxter, K.; Simonson, J.; Zinsmeister, A.R. A stable isotope breath test with a standard meal for abnormal gastric emptying of solids in the clinic and in research. Clin. Gastroenterol. Hepatol. 2008, 6, 635–643.e631. [Google Scholar] [CrossRef] [Green Version]
- Sanaka, M.; Yamamoto, T.; Kuyama, Y. Retention, fixation, and loss of the [13C] label: A review for the understanding of gastric emptying breath tests. Dig. Dis. Sci. 2008, 53, 1747–1756. [Google Scholar] [CrossRef]
- Maes, B.D.; Ghoos, Y.F.; Geypens, B.J.; Mys, G.; Hiele, M.I.; Rutgeerts, P.J.; Vantrappen, G. Combined carbon-13-glycine/carbon-14-octanoic acid breath test to monitor gastric emptying rates of liquids and solids. J. Nucl. Med. 1994, 35, 824–831. [Google Scholar] [PubMed]
- George, N.S.; Sankineni, A.; Parkman, H.P. Small intestinal bacterial overgrowth in gastroparesis. Dig. Dis. Sci. 2014, 59, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.; Andresen, V.; Wolter, J.; Layer, P.; Camilleri, M. Influence of clinical parameters on the results of 13C-octanoic acid breath tests: Examination of different mathematical models in a large patient cohort. Neurogastroenterol. Motil. 2009, 21, e1039–e1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, B.; McCallum, R.W.; Koch, K.L.; Sitrin, M.D.; Wo, J.M.; Chey, W.D.; Hasler, W.L.; Lackner, J.M.; Katz, L.A.; Semler, J.R.; et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment. Pharmacol. Ther. 2008, 27, 186–196. [Google Scholar] [CrossRef] [Green Version]
- Cassilly, D.; Kantor, S.; Knight, L.C.; Maurer, A.H.; Fisher, R.S.; Semler, J.; Parkman, H.P. Gastric emptying of a non-digestible solid: Assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol. Motil. 2008, 20, 311–319. [Google Scholar] [CrossRef]
- Lee, A.A.; Rao, S.; Nguyen, L.A.; Moshiree, B.; Sarosiek, I.; Schulman, M.I.; Wo, J.M.; Parkman, H.P.; Wilding, G.E.; McCallum, R.W.; et al. Validation of Diagnostic and Performance Characteristics of the Wireless Motility Capsule in Patients with Suspected Gastroparesis. Clin. Gastroenterol. Hepatol. 2019, 17, 1770–1779.e1772. [Google Scholar] [CrossRef]
- Kloetzer, L.; Chey, W.D.; McCallum, R.W.; Koch, K.L.; Wo, J.M.; Sitrin, M.; Katz, L.A.; Lackner, J.M.; Parkman, H.P.; Wilding, G.E.; et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogastroenterol. Motil. 2010, 22, 527–533.e117. [Google Scholar] [CrossRef] [Green Version]
- Hasler, W.L.; May, K.P.; Wilson, L.A.; Van Natta, M.; Parkman, H.P.; Pasricha, P.J.; Koch, K.L.; Abell, T.L.; McCallum, R.W.; Nguyen, L.A.; et al. Relating gastric scintigraphy and symptoms to motility capsule transit and pressure findings in suspected gastroparesis. Neurogastroenterol. Motil. 2018, 30, e13196. [Google Scholar] [CrossRef]
- Parkman, H.P.; Sharkey, E.; McCallum, R.W.; Hasler, W.L.; Koch, K.L.; Sarosiek, I.; Abell, T.L.; Kuo, B.; Shulman, R.J.; Grover, M.; et al. Constipation in Patients With Symptoms of Gastroparesis: Analysis of Symptoms and Gastrointestinal Transit. Clin. Gastroenterol. Hepatol. 2022, 20, 546–558.e545. [Google Scholar] [CrossRef]
- Zikos, T.A.; Kamal, A.N.; Neshatian, L.; Triadafilopoulos, G.; Clarke, J.O.; Nandwani, M.; Nguyen, L.A. High Prevalence of Slow Transit Constipation in Patients With Gastroparesis. J. Neurogastroenterol. Motil. 2019, 25, 267–275. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.D. Electrogastrography: Methodology, validation and applications. J. Neurogastroenterol. Motil. 2013, 19, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.D.; Schirmer, B.D.; McCallum, R.W. Serosal and cutaneous recordings of gastric myoelectrical activity in patients with gastroparesis. Am. J. Physiol. 1994, 266 Pt 1, G90–G98. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Z.; McCallum, R.W. Electrogastrography: Principles and Applications; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1994. [Google Scholar]
- Chen, J.D.; Richards, R.D.; McCallum, R.W. Identification of gastric contractions from the cutaneous electrogastrogram. Am. J. Gastroenterol. 1994, 89, 79–85. [Google Scholar] [PubMed]
- Geldof, H.; van der Schee, E.J.; Grashuis, J.L. Electrogastrographic characteristics of interdigestive migrating complex in humans. Am. J. Physiol. 1986, 250 Pt 1, G165–G171. [Google Scholar] [CrossRef] [PubMed]
- van der Schee, E.J.; Grashuis, J.L. Contraction-related, low-frequency components in canine electrogastrographic signals. Am. J. Physiol. 1983, 245, G470–G475. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.D.; Lin, Z.; Pan, J.; McCallum, R.W. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig. Dis. Sci. 1996, 41, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Gharibans, A.A.; Kim, S.; Kunkel, D.; Coleman, T.P. High-Resolution Electrogastrogram: A Novel, Noninvasive Method for Determining Gastric Slow-Wave Direction and Speed. IEEE Trans. Biomed. Eng. 2017, 64, 807–815. [Google Scholar] [CrossRef]
- Gharibans, A.A.; Coleman, T.P.; Mousa, H.; Kunkel, D.C. Spatial Patterns from High-Resolution Electrogastrography Correlate with Severity of Symptoms in Patients with Functional Dyspepsia and Gastroparesis. Clin. Gastroenterol. Hepatol. 2019, 17, 2668–2677. [Google Scholar] [CrossRef]
- Gharibans, A.A.; Hayes, T.C.L.; Carson, D.A.; Calder, S.; Varghese, C.; Du, P.; Yarmut, Y.; Waite, S.; Keane, C.; Woodhead, J.S.T.; et al. A novel scalable electrode array and system for non-invasively assessing gastric function using flexible electronics. Neurogastroenterol. Motil. 2023, 35, e14418. [Google Scholar] [CrossRef]
- Calder, S.; Schamberg, G.; Varghese, C.; Waite, S.; Sebaratnam, G.; Woodhead, J.S.T.; Du, P.; Andrews, C.N.; O’Grady, G.; Gharibans, A.A. An automated artifact detection and rejection system for body surface gastric mapping. Neurogastroenterol. Motil. 2022, 34, e14421. [Google Scholar] [CrossRef]
- Carson, D.A.; O’Grady, G.; Du, P.; Gharibans, A.A.; Andrews, C.N. Body surface mapping of the stomach: New directions for clinically evaluating gastric electrical activity. Neurogastroenterol. Motil. 2021, 33, e14048. [Google Scholar] [CrossRef] [PubMed]
- Calder, S.; Cheng, L.K.; Andrews, C.N.; Paskaranandavadivel, N.; Waite, S.; Alighaleh, S.; Erickson, J.C.; Gharibans, A.; O’Grady, G.; Du, P. Validation of noninvasive body-surface gastric mapping for detecting gastric slow-wave spatiotemporal features by simultaneous serosal mapping in porcine. Am. J. Physiol. Gastrointest. Liver Physiol. 2022, 323, G295–G305. [Google Scholar] [CrossRef]
- Boeckxstaens, G.; Camilleri, M.; Sifrim, D.; Houghton, L.A.; Elsenbruch, S.; Lindberg, G.; Azpiroz, F.; Parkman, H.P. Fundamentals of Neurogastroenterology: Physiology/Motility—Sensation. Gastroenterology 2016, 150, 1292–1304.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xing, J.H.; Chen, J.D. Gastric electrical stimulation with parameters for gastroparesis enhances gastric accommodation and alleviates distention-induced symptoms in dogs. Dig. Dis. Sci. 2006, 51, 2160–2164. [Google Scholar] [CrossRef] [PubMed]
- Ramos, G.P.; Camilleri, M. Ten controversies in gastroparesis and a look to the future. Neurogastroenterol. Motil. 2022, e14494. [Google Scholar] [CrossRef] [PubMed]
- Hjelland, I.E.; Ofstad, A.P.; Narvestad, J.K.; Berstad, A.; Hausken, T. Drink tests in functional dyspepsia: Which drink is best? Scand. J. Gastroenterol. 2004, 39, 933–937. [Google Scholar] [CrossRef]
- Sivarao, D.V.; Mashimo, H.; Goyal, R.K. Pyloric sphincter dysfunction in nNOS-/- and W/Wv mutant mice: Animal models of gastroparesis and duodenogastric reflux. Gastroenterology 2008, 135, 1258–1266. [Google Scholar] [CrossRef] [Green Version]
- Rosen, R.; Garza, J.M.; Tipnis, N.; Nurko, S. An ANMS-NASPGHAN consensus document on esophageal and antroduodenal manometry in children. Neurogastroenterol. Motil. 2018, 30, e13239. [Google Scholar] [CrossRef]
- Banerjee, S.; Pal, A.; Fox, M. Volume and position change of the stomach during gastric accommodation and emptying: A detailed three-dimensional morphological analysis based on MRI. Neurogastroenterol. Motil. 2020, 32, e13865. [Google Scholar] [CrossRef]
- Lu, K.H.; Cao, J.; Oleson, S.T.; Powley, T.L.; Liu, Z. Contrast-Enhanced Magnetic Resonance Imaging of Gastric Emptying and Motility in Rats. IEEE Trans. Biomed. Eng. 2017, 64, 2546–2554. [Google Scholar] [CrossRef]
- de Jonge, C.S.; Smout, A.; Nederveen, A.J.; Stoker, J. Evaluation of gastrointestinal motility with MRI: Advances, challenges and opportunities. Neurogastroenterol. Motil. 2018, 30, e13257. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Bredenoord, A.J.; Fox, M.; Gyawali, C.P.; Roman, S.; Smout, A.; Pandolfino, J.E.; International Working Group for Disorders of Gastrointestinal, Motility and Function. Expert consensus document: Advances in the management of oesophageal motility disorders in the era of high-resolution manometry: A focus on achalasia syndromes. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 677–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muangchan, N.; Kooptiwut, S.; Tapechum, S.; Akarasereenont, P.; Vongsopanagul, N.; Pongwattanapakin, K.; Chaikomin, R. (13)C-Acetic Acid Breath Test Monitoring of Gastric Emptying during Disease Progression in Diabetic Rats. Biol. Pharm. Bull. 2017, 40, 1506–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrit, K.; Boscan, P.; Ferguson, L.E.; Bradley, A.M.; Dowers, K.L.; Rao, S.; Twedt, D.C. Minimally invasive wireless motility capsule to study canine gastrointestinal motility and pH. Vet. J. 2017, 227, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cheung, J.Y.; Chen, J.D. Detection and deletion of motion artifacts in electrogastrogram using feature analysis and neural networks. Ann. Biomed. Eng. 1997, 25, 850–857. [Google Scholar] [CrossRef]
- Tacheci, I.; Kvetina, J.; Kunes, M.; Varayil, J.E.; Ali, S.M.; Pavlik, M.; Kopacova, M.; Rejchrt, S.; Bures, J.; Pleskot, M. Electrogastrography in experimental pigs: The influence of gastrointestinal injury induced by dextran sodium sulphate on porcine gastric erythromycin-stimulated myoelectric activity. Neuroendocrinol. Lett. 2011, 32, 131–136. [Google Scholar]
- Watts, L.S.; Baker, J.R.; Lee, A.A.; Harer, K.; Bowers, N.; Law, R.; Hasler, W.L. Impact of gastric per-oral endoscopic myotomy on static and dynamic pyloric function in gastroparesis patients. Neurogastroenterol. Motil. 2020, 32, e13892. [Google Scholar] [CrossRef]
- Desprez, C.; Melchior, C.; Wuestenberghs, F.; Zalar, A.; Jacques, J.; Leroi, A.M.; Gourcerol, G. Pyloric distensibility measurement predicts symptomatic response to intrapyloric botulinum toxin injection. Gastrointest. Endosc. 2019, 90, 754–760.e751. [Google Scholar] [CrossRef]
- Sclocco, R.; Fisher, H.; Staley, R.; Han, K.; Mendez, A.; Bolender, A.; Coll-Font, J.; Kettner, N.W.; Nguyen, C.; Kuo, B.; et al. Cine gastric MRI reveals altered Gut-Brain Axis in Functional Dyspepsia: Gastric motility is linked with brainstem-cortical fMRI connectivity. Neurogastroenterol. Motil. 2022, 34, e14396. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, J.D.Z.; Nojkov, B. Diagnostic Methods for Evaluation of Gastric Motility—A Mini Review. Diagnostics 2023, 13, 803. https://doi.org/10.3390/diagnostics13040803
Wang Y, Chen JDZ, Nojkov B. Diagnostic Methods for Evaluation of Gastric Motility—A Mini Review. Diagnostics. 2023; 13(4):803. https://doi.org/10.3390/diagnostics13040803
Chicago/Turabian StyleWang, Yan, Jiande D. Z. Chen, and Borko Nojkov. 2023. "Diagnostic Methods for Evaluation of Gastric Motility—A Mini Review" Diagnostics 13, no. 4: 803. https://doi.org/10.3390/diagnostics13040803