Clinical and Biological Data in Patients with Pancreatic Cancer vs. Chronic Pancreatitis—A Single Center Comparative Analysis
Abstract
:1. Introduction
2. Materials and Methods
- Group A: patients with pancreatic cancer stages II–IV.
- Group B: patients with risk factors for pancreatic cancer.
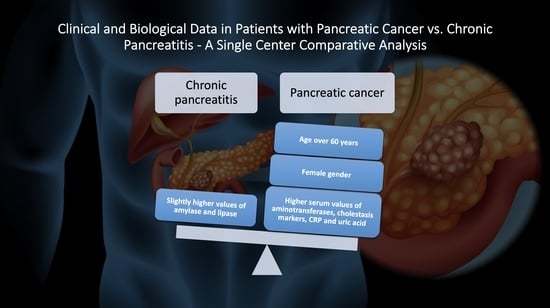
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, V.M.; Ahuja, N. Early detection of pancreatic cancer. Chin. J. Cancer Res. 2015, 27, 321–331. [Google Scholar] [PubMed]
- SEER Cancer Stat Facts: Pancreatic Cancer. Available online: https://seer.cancer.gov/statfacts/html/pancreas.html (accessed on 6 July 2022).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. GLOBOCAN Database. Available online: https://gco.iarc.fr/today/home (accessed on 6 July 2022).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Naito, Y.; Cope, L.; Naranjo-Suarez, S.; Saunders, T.; Hong, S.M.; Goggins, M.G.; Herman, J.M.; Wolfgang, C.L.; Iacobuzio-Donahue, C.A. Functional p38 MAPK Identified by Biomarker Profiling of Pancreatic Cancer Restrains Growth through JNK Inhibition and Correlates with Improved Survival. Clin. Cancer Res. 2014, 20, 6200–6211. [Google Scholar] [CrossRef] [Green Version]
- Pallag, A.; Rosca, E.; Tit, D.M.; Mutiu, G.; Bungau, S.G.; Pop, O.L. Monitoring the effects of treatment in colon cancer cells using immunohistochemical and histoenzymatic techniques. Rom. J. Morphol. Embriol. 2015, 56, 1103–1109. [Google Scholar]
- Bano, I.; Horky, P.; Abbas, S.Q.; Majid, M.; Bilal, A.H.M.; Ali, F.; Behl, T.; Hassan, S.S.U.; Bungau, S. Ferroptosis: A New Road towards Cancer Management. Molecules 2022, 27, 2129. [Google Scholar] [CrossRef]
- Mukhtar, M.; Bilal, M.; Rahdar, A.; Barani, M.; Arshad, R.; Behl, T.; Brisc, C.; Banica, F.; Bungau, S. Nanomaterials for Diagnosis and Treatment of Brain Cancer: Recent Updates. Chemosensors 2020, 8, 117. [Google Scholar] [CrossRef]
- Eissa, M.A.L.; Lerner, L.; Abdelfatah, E.; Shankar, N.; Canner, J.K.; Hasan, N.M.; Yaghoobi, V.; Huang, B.; Kerner, Z.; Takaesu, F.; et al. Promoter methylation of ADAMTS1 and BNC1 as potential biomarkers for early detection of pancreatic cancer in blood. Clin. Epigenetics 2019, 11, 59. [Google Scholar] [CrossRef]
- Van Roessel, S.; Kasumova, G.G.; Verheij, J.; Najarian, R.M.; Maggino, L.; de Pastena, M.; Malleo, G.; Marchegiani, G.; Salvia, R.; Ng, S.C.; et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg. 2018, 153, e183617. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Feng, Z.; Miao, R.; Liu, X.; Liu, C.; Liu, Z. Prognosis and survival analysis of patients with pancreatic cancer: Retrospective experience of a single institution. World J. Surg. Oncol. 2022, 20, 11. [Google Scholar] [CrossRef] [PubMed]
- Mogoanta, S.S.; Costache, A.; Mutiu, G.; Bungau, S.G.; Ghilusi, M.; Grosu, F.; Vasile, M.; Vilcea, I.D.; Gherghinescu, M.C.; Mogoanta, L.; et al. A nonfunctional neuroendocrine tumor of the pancreas—A case report. Rom. J. Morphol. Embriol. 2015, 56 (Suppl. S2), 511–519. [Google Scholar]
- Distler, M.; Rückert, F.; Hunger, M.; Kersting, S.; Pilarsky, C.; Saeger, H.D.; Grützmann, R. Evaluation of survival in patients after pancreatic head resection for ductal adenocarcinoma. BMC Surg. 2013, 13, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghe, G.; Bungau, S.; Ilie, M.; Behl, T.; Vesa, C.M.; Brisc, C.; Bacalbasa, N.; Turi, V.; Costache, R.S.; Diaconu, C. Early diagnosis of pancreatic cancer: The key for survvival. Diagnostics 2020, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, G.; Diaconu, C.C.; Ionescu, V.; Constantinescu, G.; Bacalbasa, N.; Bungau, S.; Gaman, M.A.; Stan-Ilie, M. Risk factors for pancreatic cancer: Emerging Role of viral hepatitis. J. Pers. Med. 2022, 12, 83. [Google Scholar] [CrossRef]
- Wolfgang, C.L.; Herman, J.M.; Laheru, D.A.; Klein, A.P.; Erdek, M.A.; Fishman, E.K.; Hruban, R.H. Recent progress in pancreatic cancer. CA Cancer J. Clin. 2013, 63, 318–348. [Google Scholar] [CrossRef] [Green Version]
- Mario, C.; Marilisa, F.; Kryssia, I.R.C.; Pellegrino, C.; Ginevra, C.; Chiara, M.; Alberto, B.; Antonio, N.; Gioacchino, L.; Tiziana, M.; et al. Epidemiology and risk factors of pancreatic cancer. Acta Biomed. 2018, 89, 141–146. [Google Scholar]
- Han, M.; Tran, T.P.T.; Oh, J.K. Chronic pancreatitis and cancer risk in a matched cohort study using national claims data in South Korea. Sci. Rep. 2022, 12, 5545. [Google Scholar] [CrossRef]
- Xiao, A.Y.X.; Tan, M.L.Y.; Wu, L.M.; Asrani, V.M.; Windsor, J.A.; Yadav, D.; Petrov, M.S. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol. Hepatol. 2016, 1, 45–55. [Google Scholar] [CrossRef]
- Ouyang, G.; Pan, G.; Liu, Q.; Wu, Y.; Liu, Z.; Lu, W.; Li, S.; Zhou, Z.; Wen, Y. The global, regional, and national burden of pancreatitis in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. BMC Med. 2020, 18, 388. [Google Scholar] [CrossRef]
- Olesen, S.S.; Mortensen, L.H.; Zinck, E.; Becker, U.; Drewes, A.M.; Nojgaard, C.; Novovic, S.; Yadav, D.; Tolstrup, J.S. Time trends in incidence and prevalence of chronic pancreatitis: A 25-year population-based nationwide study. United Eur. Gastroenterol. J. 2020, 9, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kichler, A.; Jang, S. Chronic Pancreatitis: Epidemiology, Diagnosis, and Management Updates. Drugs 2020, 80, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Kirkegard, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic pancreatitis and pancreatic cancer risk: A systematic review and meta-analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Ekbom, A.; McLaughlin, J.K.; Karlsson, B.M.; Nyren, O.; Gridley, G.; Adami, H.O.; Fraumeni, J.F. Pancreatitis and pancreatic cancer: A population-based study. J. Natl. Cancer Inst. 1994, 86, 625. [Google Scholar] [CrossRef]
- Javadrashid, D.; Baghbanzadeh, A.; Derakhshani, A.; Leone, P.; Silvestris, N.; Racanelli, V.; Solimando, A.G.; Baradaran, B. Pancreatic Cancer Signaling Pathways, Genetic Alterations, and Tumor Microenvironment: The Barriers Affecting the Method of Treatment. Biomedicines 2021, 9, 373. [Google Scholar] [CrossRef]
- Munigala, S.; Kanwal, F.; Xian, H.; Agarwal, B. New diagnosis of chronic pancreatitis: Risk of missing an underlying pancreatic cancer. Am. J. Gastroenterol. 2014, 109, 1824–1830. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2020. Available online: https://www.R-project.org/ (accessed on 10 May 2022).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Oaks, T.; Fox, J. Effect Displays in R for Generalised Linear Models. J. Stat. Softw. 2003, 8, 1–27. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 10 May 2022).
- Sjoberg, D.D.; Curry, M.; Hannum, M.; Larmarange, J.; Whiting, K.; Zabor, E.C. gtsummary: Presentation-Ready Data Summary and Analytic Result Tables. R Package Version 1.4.2. Available online: https://CRAN.R-project.org/package=gtsummary (accessed on 10 May 2022).
- Lee, Y. logisticRR: Adjusted Relative Risk from Logistic Regression. R Package Version 0.3.0. 2020. Available online: https://CRAN.R-project.org/package=logisticRR (accessed on 10 May 2022).
- De La Cruz, M.S.; Young, A.P.; Ruffin, M.T. Diagnosis and management of pancreatic cancer. Am. Fam. Physician 2014, 89, 626–632. [Google Scholar]
- Porta, M.; Fabregat, X.; Malats, N.; Guarner, L.; Carrato, A.; de Miguel, A.; Ruiz, L.; Jariod, M.; Costafreda, S.; Coll, S.; et al. Exocrine pancreatic cancer: Symptoms at presentation and their relation to tumour site and stage. Clin. Transl. Oncol. 2005, 7, 189–197. [Google Scholar] [CrossRef]
- Wang, M.; Gorelick, F.; Bhargava, A. Sex differences in the exocrine pancreas and associated diseases. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 427–441. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamada, T.; Hashimoto, T.; Takahashi, S.; Sogawa, M.; Ohara, H.; Nakazawa, T.; Sano, H.; Kuno, A.; Joh, T.; et al. Estradiol alleviates acinar cell apoptosis and chronic pancreatitis in male Wistar Bonn/Kobori rats. Pancreas 2003, 26, e59–e66. [Google Scholar] [CrossRef] [PubMed]
- Diakopoulos, K.N.; Lesina, M.; Wörmann, S.; Song, L.; Aichler, M.; Schild, L.; Artati, A.; Römisch-Margl, W.; Wartmann, T.; Fischer, R.; et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology 2015, 148, 626–638.e17. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.N.; Sengupta, K.; Banerjee, S.; Saxena, N.K.; Banerjee, S.K. 2-Methoxyestradiol exhibits a biphasic effect on VEGF-A in tumor cells and upregulation is mediated through ER-alpha: A possible signaling pathway associated with the impact of 2-ME2 on proliferative cells. Neoplasia 2003, 5, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skipworth, R.J.; Moses, A.G.; Sangster, K.; Sturgeon, C.M.; Voss, A.C.; Fallon, M.T.; Anderson, R.A.; Ross, J.A.; Fearon, K.C.H. Interaction of gonadal status with systemic inflammation and opioid use in determining nutritional status and prognosis in advanced pancreatic cancer. Support. Care Cancer 2011, 19, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Catalano, M.; Aprile, G.; Conca, R.; Petroli, R.; Ramello, M.; Roviello, G. The impact of age, performance status and comorbidities on nab-paclitaxel plus gemcitabine effectiveness in patients with metastatic pancreatic cancer. Sci. Rep. 2022, 12, 8244. [Google Scholar] [CrossRef]
- Schneider, A.; Lohr, J.M.; Singer, M.V. The M-ANNHEIM classification of chronic pancreatitis: Introduction of a unifying classification system based on a review of previous classifications of the disease. J. Gastroenterol. 2007, 42, 101–119. [Google Scholar] [CrossRef]
- Nair, R.J.; Lawler, L.; Miller, M.R. Chronic pancreatitis. Am. Fam. Physician 2007, 76, 1679–1688. [Google Scholar]
- Wittel, U.A.; Pandey, K.K.; Andrianifahanana, M.; Johansson, S.L.; Cullen, D.M.; Akhter, M.P.; Brand, R.E.; Prokopczyk, B.; Batra, S.K. Chronic Pancreatic Inflammation Induced by Environmental Tobacco Smoke Inhalation in Rats. Am. J. Gastroenterol. 2006, 101, 148–159. [Google Scholar] [CrossRef]
- Edderkaoui, M.; Thrower, E. Smoking and pancreatic disease. J. Cancer Ther. 2013, 4, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Askari, M.D.; Tsao, M.S.; Cekanova, M.; Schuller, H.M. Ethanol and the Tobacco-Specific Carcinogen, NNK, Contribute to Signaling in Immortalized Human Pancreatic Duct Epithelial Cells. Pancreas 2006, 33, 53–62. [Google Scholar] [CrossRef]
- Ye, W.; Lagergren, J.; Weiderpass, E.; Nyren, O.; Adami, H.O.; Ekbom, A. Alcohol abuse and the risk of pancreatic cancer. Gut 2002, 51, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Pacncreatic Cancer Risk Factors. Available online: www.cancer.org (accessed on 23 July 2022).
- Masamune, A.; Nabeshima, T.; Kikuta, K.; Hamada, S.; Nakano, E.; Kume, K.; Kanno, A.; Sato, A.; Tachibana, Y.; Inatomi, O.; et al. Prospective study of early chronic pancreatitis diagnosed based on the Japanese diagnostic criteria. J. Gastroenterol. 2019, 54, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Yamamiya, A.; Tominaga, K.; Hoshi, K.; Nagashima, K.; Minaguchi, T.; Haruyama, Y.; Irisawa, A. The Risk Factors for Progression to Chronic Pancreatitis in Patients with Past-History of Acute Pancreatitis: A Retrospective Analysis Based on Mechanistic Definition. J. Clin. Med. 2022, 11, 2209. [Google Scholar] [CrossRef]
- Nojgaard, C.; Bcker, U.; Matzen, P.; Andersen, J.R.; Holst, C.; Bendtsen, F. Progression from acute to chronic pancreatitis: Prognostic factors, mortality, and natural course. Pancreas 2011, 40, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Houg, D.S.; Bijlsma, M.F. The hepatic pre-metastatic niche in pancreatic ductal adenocarcinoma. Mol. Cancer 2018, 17, 95. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.; Koder, S.; Kornek, G.; Pabinger, I.; Ay, C. Microparticle-associated tissue factor activity in patients with metastatic pancreatic cancer and its effect on fibrin clot formation. Transl. Res. 2014, 163, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Geddings, J.E.; Hisada, Y.; Boulaftali, Y.; Getz, T.M.; Whelihan, M.; Fuentes, R.; Dee, R.; Cooley, B.C.; Key, N.S.; Wolberg, A.S.; et al. Tissue factor-positive tumor microvesicles activate platelets and enhance thrombosis in mice. J. Thromb. Haemost. 2016, 4, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Pezzilli, R.; d’Eril, G.M.; Barassi, A. Can Serum Pancreatic Amylase and Lipase Levels Be Used as Diagnostic Markers to Distinguish between Patients with Mucinous Cystic Lesions of the Pancreas, Chronic Pancreatitis, and Pancreatic Ductal Adenocarcinoma? Pancreas 2016, 45, 1272–1275. [Google Scholar] [CrossRef]
- Weiss, F.U.; Schurmann, C.; Guenther, A.; Ernst, F.; Teumer, A.; Mayerle, J.; Simon, P.; Volzke, H.; Radke, D.; Greinacher, A.; et al. Fucosyltransferase 2 (FUT2) non-secretor status and blood group B are associated with elevated serum lipase activity in asymptomatic subjects, and an increased risk for chronic pancreatitis: A genetic association study. Gut 2015, 64, 646–656. [Google Scholar] [CrossRef]
- Cardillo, N.; Seible, D.M.; Fero, K.E.; Bruggeman, A.R.; Sarkar, R.R.; Azuara, A.; Simpsonet, D.R.; Murphy, J.D. Clinical Impact of Local Progression in Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2018, 16, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Iacobuzio-Donahue, C.A.; Fu, B.; Yachida, S.; Luo, M.; Abe, H.; Henderson, C.M.; Vilardell, F.; Wang, Z.; Keller, J.W.; Banerjee, P.; et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pan- creatic cancer. J. Clin. Oncol. 2009, 27, 1806–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silovs, A.; Strumfa, I.; Riekstins, R.; Vanags, A.; Gardovskis, J. Systemic Inflammatory Response in Pancreatic Ductal Adenocarcinoma. Advances in Pancreatic Cancer. IN Tech Open 2018. Available online: https://www.intechopen.com/books/6740 (accessed on 20 August 2022).
- Argilés, J.M.; Busquets, S.; López-Soriano, F.J. Cytokines in the pathogenesis of cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, A.; Onoda, H.; Imai, N.; Iwaku, A.; Oishi, M.; Fushiya, N.; Koike, K.; Nishino, H.; Tajiri, H. Comparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinoma. Br. J. Cancer 2012, 107, 988–993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elinav, E.; Nowarski, R.; Thaiss, C.A.; Hu, B.; Jin, C.; Flavell, R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer 2013, 13, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Hua, Y.Q.; Wang, D.; Chen, L.Y.; Wu, C.J.; Chen, Z.; Liu, L.M.; Chen, H. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J. Transl. Med. 2019, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, A.M.; Mustonen, H.K.; Stenman, U.H.; Seppanen, H.E.; Haglund, C.H. Combining CRP and CA19-9 in a novel prognostic score in pancreatic ductal adenocarcinoma. Sci. Rep. 2021, 11, 781. [Google Scholar] [CrossRef]
- Basso, D.; Fabris, C.; Meani, A.; Del Favero, G.; Vianello, D.; Angonesse, C.; Meggiato, T.; Bellinvia, S.; Fogar, P.; Petrin, P. C reactive protein in pancreatic cancer and chronic pancreatitis. Ann. Clin. Res. 1988, 20, 414–416. [Google Scholar]
- Greer, J.B.; Greer, P.; Sandhu, B.S.; Alkaade, S.; Wilcox, C.M.; Anderson, M.A.; Sherman, S.; Gardner, T.B.; Lewis, M.D.; Guda, N.M.; et al. Nutrition and inflammatory biomarkers in chronic pancreatitis patients. Nutr. Clin. Pract. 2019, 34, 387–399. [Google Scholar] [CrossRef]
- Fini, M.A.; Elias, A.; Johnson, R.J.; Wright, R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012, 1, 16. [Google Scholar] [CrossRef] [Green Version]
- Sahin, I.H.; Hassan, M.M.; Garrett, C.R. Impact of non-steroidal anti-inflammatory drugs on gastrointestinal cancers: Current state-of-the science. Cancer Lett. 2014, 345, 249–257. [Google Scholar] [CrossRef]
- Oshima, H.; Oshima, M. The inflammatory network in the gastrointestinal tumor microenvironment: Lessons from mouse models. J. Gastroenterol. 2012, 47, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xu, P.; Liu, K.; Lin, S.; Wang, M.; Tian, T.; Dai, C.; Deng, Y.; Li, N.; Hao, Q.; et al. Hyperuricemia and gout are associated with cancer incidence and mortality: A meta-analysis based on cohort studies. J. Cell Physiol. 2019, 234, 14364–14376. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Huang, J.J.; Mi, N.N.; Lin, Y.Y.; He, Q.S.; Lu, Y.W.; Yue, P.; Bai, B.; Zhang, J.-D.; Zhang, C.; et al. Association between serum acid and hepatobiliary-pancreatic cancer: A cohort study. World J. Gastroenterol. 2020, 26, 7061–7075. [Google Scholar] [CrossRef] [PubMed]
- Poruk, K.E.; Gay, D.Z.; Brown, K.; Mulvihill, J.D.; Boucher, K.M.; Scaife, C.L.; Firpo, M.A.; Mulvihill, S.J. The clinical utility of CA19-9 in pancreatic adenocarcinoma: Diagnostic and prognostic updates. Curr. Mol. Med. 2013, 13, 340–351. [Google Scholar] [PubMed]
- Lee, K.J.; Yi, S.W.; Chung, M.J.; Park, S.W.; Song, S.Y.; Chung, J.B.; Park, J.Y. Serum CA 19-9 and CEA levels as a prognostic factor in pancreatic adenocarcinoma. Yonsei Med. J. 2013, 54, 643–649. [Google Scholar] [CrossRef]









| Predictor | N | Chronic Pancreatitis (n = 60) | Pancreatic Cancer (n = 60) | OR (95% CI) | p |
|---|---|---|---|---|---|
| Sex | 120 | ||||
| Female | 16 | 36 | - | ||
| Male | 44 | 24 | 0.24 (0.08, 0.70) | 0.011 | |
| Age | 120 | 1.12 (1.06, 1.21) | <0.001 | ||
| Age groups | 120 | ||||
| <60 years | 44 | 14 | - | ||
| ≥60 years | 16 | 46 | 9.04 (2.94, 31.2) | <0.001 | |
| BMI | 120 | 60 | 60 | 1.10 (0.98, 1.25) | 0.111 |
| Smoker | 120 | ||||
| Yes | 50 | 34 | - | ||
| No | 10 | 26 | 3.82 (1.20, 13.8) | 0.029 | |
| Alcohol consumption | 120 | ||||
| Yes | 52 | 34 | - | ||
| No | 8 | 26 | 4.97 (1.48, 20.0) | 0.014 | |
| History of acute pancreatitis | 120 | ||||
| Yes | 50 | 6 | - | ||
| No | 10 | 54 | 45.0 (11.1, 251) | <0.001 | |
| History of diabetes mellitus type II | 120 | ||||
| Yes | 44 | 24 | - | ||
| No | 16 | 36 | 0.55 (0.18, 1.60) | 0.276 | |
| History of biliary lithiasis | 120 | ||||
| Yes | 40 | 18 | - | ||
| No | 20 | 42 | 1.17 (0.39, 3.52) | 0.781 | |
| Familial history of diabetes mellitus type II | 120 | ||||
| Yes | 20 | 16 | - | ||
| No | 40 | 44 | 1.37 (0.45, 4.270) | 0.574 | |
| Familial history of cancers | 120 | ||||
| Yes | 14 | 20 | - | ||
| No | 46 | 40 | 0.61 (0.19, 1.88) | 0.392 | |
| OR = odds ratio; CI = confidence interval; BMI = body mass index. | |||||
| Biological Parameters | N | Group A; N = 60 | Group B; N = 60 | p1 |
|---|---|---|---|---|
| Hemoglobin, Median (IQR) | 120 | 12.35 (11.55–13.38) | 12.80 (11.68–13.88) | 0.859 |
| Leukocytes, Median (IQR) | 120 | 7.660 (6.322–9.608) | 7.635 (6.240–9.925) | 0.824 |
| Platelets, Median (IQR) | 120 | 241.000 (202.000–298.000) | 294.000 (225.000–366.000) | 0.099 |
| Glycemia, Median (IQR) | 120 | 117 (101–134) | 102 (92–124) | 0.322 |
| Total cholesterol, Median (IQR) | 120 | 173 (134–214) | 168 (149–184) | 0.959 |
| Triglycerides, Median (IQR) | 120 | 135 (111–193) | 120 (84–148) | 0.072 |
| Aspartate aminotransferase (AST), Median (IQR) | 120 | 139 (27–196) | 26 (18–73) | 0.005 |
| Alanine aminotransferase (ALT), Median (IQR) | 120 | 100 (37–392) | 34 (19–82) | 0.006 |
| Lipase, Median (IQR) | 120 | 88 (46–358) | 395 (89–1015) | 0.029 |
| Amylase, Median (IQR) | 120 | 55 (44–101) | 116 (58–170) | 0.020 |
| Total bilirubin, Median (IQR) | 120 | 4 (1–16) | 1 (0–1) | <0.001 |
| Direct bilirubin, Median (IQR) | 120 | 3 (0.40–12) | 0.40 (0.20–1) | <0.001 |
| Gamma-glutamyl transferase (GGT), Median (IQR) | 120 | 188 (106–806) | 116 (46–359) | 0.078 |
| Alkaline phosphatase, Median (IQR) | 120 | 348 (149–518) | 132 (78–268) | 0.030 |
| C-reactive protein, Median (IQR) | 120 | 33 (23–42) | 17 (7–39) | 0.049 |
| Erythrocyte sedimentation rate, Median (IQR) | 120 | 34 (28–40) | 29 (21–57) | 0.830 |
| Uric acid, Median (IQR) | 120 | 5.75 (4.85–6.27) | 4.65 (3.85–5.32) | 0.001 |
| Carcinoembryonic antigen (CEA), Median (IQR) | 24 | 4 (2–20) | 2 (1–2) | 0.073 |
| Carbohydrate antigen 19-9 (CA 19-9), Median (IQR) | 28 | 849 (55–950) | 12 (8–57) | 0.096 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, G.; Ionescu, V.A.; Moldovan, H.; Diaconu, C.C. Clinical and Biological Data in Patients with Pancreatic Cancer vs. Chronic Pancreatitis—A Single Center Comparative Analysis. Diagnostics 2023, 13, 369. https://doi.org/10.3390/diagnostics13030369
Gheorghe G, Ionescu VA, Moldovan H, Diaconu CC. Clinical and Biological Data in Patients with Pancreatic Cancer vs. Chronic Pancreatitis—A Single Center Comparative Analysis. Diagnostics. 2023; 13(3):369. https://doi.org/10.3390/diagnostics13030369
Chicago/Turabian StyleGheorghe, Gina, Vlad Alexandru Ionescu, Horatiu Moldovan, and Camelia Cristina Diaconu. 2023. "Clinical and Biological Data in Patients with Pancreatic Cancer vs. Chronic Pancreatitis—A Single Center Comparative Analysis" Diagnostics 13, no. 3: 369. https://doi.org/10.3390/diagnostics13030369