Serum β-Defensin 2, A Novel Biomarker for the Diagnosis of Acute Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Laboratory Measurements
2.3. hIL-6, hBD1 and hBD2 ELISA
2.4. Procalcitonin ELISA
2.5. Nephelometric CRP Assay
2.6. Blood Count, Blood Chemistry Tests and Blood Coagulation Measurements
2.7. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
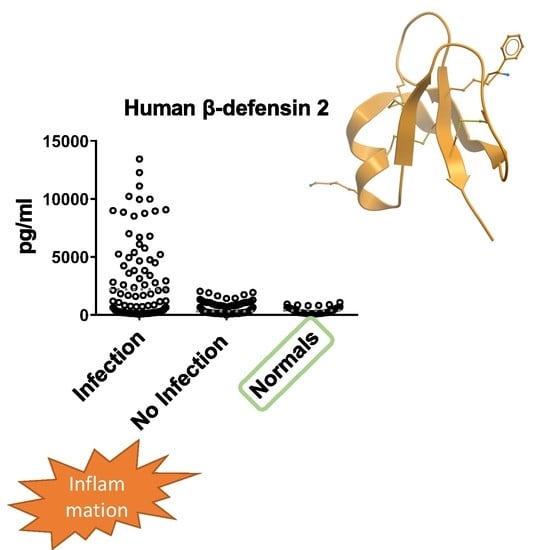
3.2. hBD2 Is Increased in Patients with Inflammation of Infectious Etiology
3.3. Predictive Ability of hBD2 for Infection
3.4. hBD1 and hBD2 Levels in Patients with Cancer
3.5. hBD1 and hBD2 Levels in Different Types of Infection
3.6. Serum hBD2 Significantly Correlates with the Levels of IL-6
3.7. Kinetic Analysis of hBD2 and CRP in Patients’ Sera Collected at Different Timepoints
3.8. Analysis of hBD2 Kinetics in Specific Cases
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahlenberg, J.M.; Kaplan, M.J. Little peptide, big effects: The role of LL-37 in inflammation and autoimmune disease. J. Immunol. 2013, 191, 4895–4901. [Google Scholar] [CrossRef] [PubMed]
- Beisswenger, C.; Bals, R. Functions of antimicrobial peptides in host defense and immunity. Curr. Protein Pept. Sci. 2005, 6, 255–264. [Google Scholar] [CrossRef]
- Toke, O. Antimicrobial peptides: New candidates in the fight against bacterial infections. Biopolymers 2005, 80, 717–735. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Izadpanah, A.; Gallo, R.L. Antimicrobial peptides. J. Am. Acad. Dermatol. 2005, 52, 381–390; quiz 391–382. [Google Scholar] [CrossRef] [PubMed]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Harder, J.; Schroder, J.M. Antimicrobial peptides in human skin. Chem. Immunol. Allergy 2005, 86, 22–41. [Google Scholar] [CrossRef]
- Thomma, B.P.; Cammue, B.P.; Thevissen, K. Plant defensins. Planta 2002, 216, 193–202. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hooper, L.V. Antimicrobial defense of the intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef]
- Muniz, L.R.; Knosp, C.; Yeretssian, G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front. Immunol. 2012, 3, 310. [Google Scholar] [CrossRef]
- Ouellette, A.J. Paneth cell alpha-defensins: Peptide mediators of innate immunity in the small intestine. Springer Semin. Immunopathol. 2005, 27, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Ouchi, Y. Antimicrobial peptide defensin: Identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases. Proc. Jpn. Academy. Ser. B Phys. Biol. Sci. 2012, 88, 152–166. [Google Scholar] [CrossRef]
- Pazgier, M.; Hoover, D.M.; Yang, D.; Lu, W.; Lubkowski, J. Human beta-defensins. Cell. Mol. Life Sci. CMLS 2006, 63, 1294–1313. [Google Scholar] [CrossRef] [PubMed]
- Mallow, E.B.; Harris, A.; Salzman, N.; Russell, J.P.; DeBerardinis, R.J.; Ruchelli, E.; Bevins, C.L. Human enteric defensins. Gene structure and developmental expression. J. Biol. Chem. 1996, 271, 4038–4045. [Google Scholar] [CrossRef]
- Ouellette, A.J.; Lualdi, J.C. A novel mouse gene family coding for cationic, cysteine-rich peptides. Regulation in small intestine and cells of myeloid origin. J. Biol. Chem. 1990, 265, 9831–9837. [Google Scholar] [CrossRef]
- Lay, F.T.; Anderson, M.A. Defensins--components of the innate immune system in plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef]
- Lehrer, R.I.; Ganz, T. Defensins of vertebrate animals. Curr. Opin. Immunol. 2002, 14, 96–102. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Yuan, J.; Osapay, G.; Osapay, K.; Tran, D.; Miller, C.J.; Ouellette, A.J.; Selsted, M.E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science 1999, 286, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Zeya, H.I.; Spitznagel, J.K. Cationic proteins of polymorphonuclear leukocyte lysosomes. II. Composition, properties, and mechanism of antibacterial action. J. Bacteriol. 1966, 91, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Zeya, H.I.; Spitznagel, J.K. Antibacterial and Enzymic Basic Proteins from Leukocyte Lysosomes: Separation and Identification. Science 1963, 142, 1085–1087. [Google Scholar] [CrossRef]
- Schutte, B.C.; McCray, P.B., Jr. [beta]-defensins in lung host defense. Annu. Rev. Physiol. 2002, 64, 709–748. [Google Scholar] [CrossRef] [PubMed]
- Mackewicz, C.E.; Yuan, J.; Tran, P.; Diaz, L.; Mack, E.; Selsted, M.E.; Levy, J.A. alpha-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS 2003, 17, F23–F32. [Google Scholar] [CrossRef]
- Chalifour, A.; Jeannin, P.; Gauchat, J.F.; Blaecke, A.; Malissard, M.; N’Guyen, T.; Thieblemont, N.; Delneste, Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood 2004, 104, 1778–1783. [Google Scholar] [CrossRef]
- Obata-Onai, A.; Hashimoto, S.; Onai, N.; Kurachi, M.; Nagai, S.; Shizuno, K.; Nagahata, T.; Matsushima, K. Comprehensive gene expression analysis of human NK cells and CD8(+) T lymphocytes. Int. Immunol. 2002, 14, 1085–1098. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial peptides of vertebrates. Comptes Rendus Biol. 2004, 327, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T. Defensins and other antimicrobial peptides: A historical perspective and an update. Comb. Chem. High Throughput Screen. 2005, 8, 209–217. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Uversky, V.N.; Redwan, E.M. Human consensus interferons: Bridging the natural and artificial cytokines with intrinsic disorder. Cytokine Growth Factor Rev. 2015, 26, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Biragyn, A.; Kwak, L.W.; Oppenheim, J.J. Mammalian defensins in immunity: More than just microbicidal. Trends Immunol. 2002, 23, 291–296. [Google Scholar] [CrossRef]
- Selsted, M.E.; Tang, Y.Q.; Morris, W.L.; McGuire, P.A.; Novotny, M.J.; Smith, W.; Henschen, A.H.; Cullor, J.S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J. Biol. Chem. 1993, 268, 6641–6648. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides in health and disease. N. Engl. J. Med. 2002, 347, 1199–1200. [Google Scholar] [CrossRef]
- Bensch, K.W.; Raida, M.; Magert, H.J.; Schulz-Knappe, P.; Forssmann, W.G. hBD-1: A novel beta-defensin from human plasma. FEBS letters 1995, 368, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Ganz, T.; Oren, A.; Lehrer, R.I. Defensins: Microbicidal and cytotoxic peptides of mammalian host defense cells. Med. Microbiol. Immunol. 1992, 181, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Selsted, M.E.; Ouellette, A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005, 6, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Valore, E.V.; Park, C.H.; Quayle, A.J.; Wiles, K.R.; McCray, P.B., Jr.; Ganz, T. Human beta-defensin-1: An antimicrobial peptide of urogenital tissues. J. Clin. Investig. 1998, 101, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- McCray, P.B., Jr.; Bentley, L. Human airway epithelia express a beta-defensin. Am. J. Respir. Cell Mol. Biol. 1997, 16, 343–349. [Google Scholar] [CrossRef]
- Goldman, M.J.; Anderson, G.M.; Stolzenberg, E.D.; Kari, U.P.; Zasloff, M.; Wilson, J.M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell 1997, 88, 553–560. [Google Scholar] [CrossRef]
- Harder, J.; Bartels, J.; Christophers, E.; Schroder, J.M. A peptide antibiotic from human skin. Nature 1997, 387, 861. [Google Scholar] [CrossRef]
- Singh, P.K.; Jia, H.P.; Wiles, K.; Hesselberth, J.; Liu, L.; Conway, B.A.; Greenberg, E.P.; Valore, E.V.; Welsh, M.J.; Ganz, T.; et al. Production of beta-defensins by human airway epithelia. Proc. Natl. Acad. Sci. USA 1998, 95, 14961–14966. [Google Scholar] [CrossRef]
- Liu, L.; Wang, L.; Jia, H.P.; Zhao, C.; Heng, H.H.; Schutte, B.C.; McCray, P.B., Jr.; Ganz, T. Structure and mapping of the human beta-defensin HBD-2 gene and its expression at sites of inflammation. Gene 1998, 222, 237–244. [Google Scholar] [CrossRef]
- Bals, R.; Wang, X.; Wu, Z.; Freeman, T.; Bafna, V.; Zasloff, M.; Wilson, J.M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J. Clin. Investig. 1998, 102, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.E. Basic principles of ROC analysis. Semin. Nucl. Med. 1978, 8, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Baltz, M.L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv. Immunol. 1983, 34, 141–212. [Google Scholar] [PubMed]
- Vijayan, A.L.; Vanimaya; Ravindran, S.; Saikant, R.; Lakshmi, S.; Kartik, R. Procalcitonin: A promising diagnostic marker for sepsis and antibiotic therapy. J. Intensive Care 2017, 5, 51. [Google Scholar] [CrossRef]
- Sinha, M.; Desai, S.; Mantri, S.; Kulkarni, A. Procalcitonin as an adjunctive biomarker in sepsis. Indian J. Anaesth. 2011, 55, 266–270. [Google Scholar] [CrossRef]
- Velissaris, D.; Pintea, M.; Pantzaris, N.; Spatha, E.; Karamouzos, V.; Pierrakos, C.; Karanikolas, M. The Role of Procalcitonin in the Diagnosis of Meningitis: A Literature Review. J. Clin. Med. 2018, 7, 148. [Google Scholar] [CrossRef]
- Luaces-Cubells, C.; Mintegi, S.; Garcia-Garcia, J.J.; Astobiza, E.; Garrido-Romero, R.; Velasco-Rodriguez, J.; Benito, J. Procalcitonin to detect invasive bacterial infection in non-toxic-appearing infants with fever without apparent source in the emergency department. Pediatr. Infect Dis. J. 2012, 31, 645–647. [Google Scholar] [CrossRef]
- Mozeika, E.; Pilmane, M.; Kisis, J. Distribution of human B-defensin 2, TNF-alpha, IL-1 alpha, IL-6 and IL-8 in psoriatic skin. Pap. Anthropol. 2011, 20, 289–302. [Google Scholar] [CrossRef]
- Boniotto, M.; Jordan, W.J.; Eskdale, J.; Tossi, A.; Antcheva, N.; Crovella, S.; Connell, N.D.; Gallagher, G. Human beta-defensin 2 induces a vigorous cytokine response in peripheral blood mononuclear cells. Antimicrob. Agents Chemother. 2006, 50, 1433–1441. [Google Scholar] [CrossRef]
- van Wetering, S.; Sterk, P.J.; Rabe, K.F.; Hiemstra, P.S. Defensins: Key players or bystanders in infection, injury, and repair in the lung? J. Allergy Clin. Immunol. 1999, 104, 1131–1138. [Google Scholar] [CrossRef]
- Diamond, G.; Kaiser, V.; Rhodes, J.; Russell, J.P.; Bevins, C.L. Transcriptional regulation of beta-defensin gene expression in tracheal epithelial cells. Infect. Immun. 2000, 68, 113–119. [Google Scholar] [CrossRef]
- Opitz, B.; Vinzing, M.; van Laak, V.; Schmeck, B.; Heine, G.; Gunther, S.; Preissner, R.; Slevogt, H.; N’Guessan, P.D.; Eitel, J.; et al. Legionella pneumophila induces IFNbeta in lung epithelial cells via IPS-1 and IRF3, which also control bacterial replication. J. Biol. Chem. 2006, 281, 36173–36179. [Google Scholar] [CrossRef] [PubMed]
- Ashitani, J.; Mukae, H.; Hiratsuka, T.; Nakazato, M.; Kumamoto, K.; Matsukura, S. Plasma and BAL fluid concentrations of antimicrobial peptides in patients with Mycobacterium avium-intracellulare infection. Chest 2001, 119, 1131–1137. [Google Scholar] [CrossRef]
- Hiratsuka, T.; Nakazato, M.; Date, Y.; Ashitani, J.; Minematsu, T.; Chino, N.; Matsukura, S. Identification of human beta-defensin-2 in respiratory tract and plasma and its increase in bacterial pneumonia. Biochem. Biophys. Res. Commun. 1998, 249, 943–947. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; He, L.R.; Wang, W.; Wang, G.H.; He, Z.Y. Prognostic value of plasma human beta-defensin 2 level on short-term clinical outcomes in patients with community-acquired pneumonia: A preliminary study. Respir. Care 2013, 58, 655–661. [Google Scholar] [CrossRef]
- Donald, C.D.; Sun, C.Q.; Lim, S.D.; Macoska, J.; Cohen, C.; Amin, M.B.; Young, A.N.; Ganz, T.A.; Marshall, F.F.; Petros, J.A. Cancer-specific loss of beta-defensin 1 in renal and prostatic carcinomas. Lab Investig. 2003, 83, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Skrygan, M.; Huyn, J.; Bechara, F.G.; Sand, M.; Altmeyer, P.; Kreuter, A. Pattern of mRNA expression of beta-defensins in basal cell carcinoma. BMC Cancer 2006, 6, 163. [Google Scholar] [CrossRef]
- Joly, S.; Compton, L.M.; Pujol, C.; Kurago, Z.B.; Guthmiller, J.M. Loss of human beta-defensin 1, 2, and 3 expression in oral squamous cell carcinoma. Oral Microbiol. Immunol. 2009, 24, 353–360. [Google Scholar] [CrossRef]
- Schuetz, A.N.; Yin-Goen, Q.; Amin, M.B.; Moreno, C.S.; Cohen, C.; Hornsby, C.D.; Yang, W.L.; Petros, J.A.; Issa, M.M.; Pattaras, J.G.; et al. Molecular classification of renal tumors by gene expression profiling. J. Mol. Diagn. 2005, 7, 206–218. [Google Scholar] [CrossRef]
- Arimura, Y.; Ashitani, J.; Yanagi, S.; Tokojima, M.; Abe, K.; Mukae, H.; Nakazato, M. Elevated serum beta-defensins concentrations in patients with lung cancer. Anticancer Res. 2004, 24, 4051–4057. [Google Scholar]
- Winter, J.; Pantelis, A.; Reich, R.; Martini, M.; Kraus, D.; Jepsen, S.; Allam, J.P.; Novak, N.; Wenghoefer, M. Human beta-defensin-1, -2, and -3 exhibit opposite effects on oral squamous cell carcinoma cell proliferation. Cancer Invest. 2011, 29, 196–201. [Google Scholar] [CrossRef] [PubMed]








| Variables | Patients | Infection | No Infection | Healthy Individuals |
|---|---|---|---|---|
| Number of individuals | 114 | 42 | 72 | 46 |
| Number of samples | 423 | 116 | 307 | 46 |
| Serial samples/patient | 3.7 | 2.8 | 4.3 | N/A |
| Age (years) ± SD | 66 ± 18 | 69 ± 3 | 65 ± 2 | 64 ± 20 |
| Female/Male | 68/46 | 25/17 | 42/30 | 28/18 |
| CRP (mg/dL) ± SD | 12.1 ± 9.6 | 14.3 ± 11.4 | 11.3 ± 8.5 | 0.2 ± 0.16 |
| hBD1 (pg/mL) ± SD | 2942 ± 1876 | 3337 ± 1884 | 3059 ± 1810 | 2858 ± 2020 |
| hBD2 (pg/mL) ± SD | 853.1 ± 1018 | 2304.0 ± 2071 | 311.9 ± 247.9 | 378.2 ± 281.1 |
| PCT (ng/mL) ± SD | 112.8 ± 165.3 | 150.6 ± 197.0 | 86.8 ± 90.0 | 0.6 ± 0.7 |
| WBC (K/μL) ± SD | 10.7 ± 5.6 | 11.4 ± 6.7 | 10.4 ± 4.8 | 6.3 ± 3.5 |
| NEU (K/μL) ± SD | 8.4 ± 4.8 | 8.9 ± 5.4 | 8.2 ± 4.4 | 3.9 ± 2.7 |
| Number of Patients | Number of Samples | |
|---|---|---|
| Urinary tract infection | 9 | 20 |
| Wound/tissue infection | 7 | 23 |
| Respiratory infection | 12 | 37 |
| Blood infection | 5 | 9 |
| Sputum infection | 3 | 6 |
| Gastrointestinal infection | 4 | 18 |
| Other types of infection | 2 | 3 |
| Infection total | 42 | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Routsias, J.G.; Marinou, D.; Mavrouli, M.; Tsakris, A.; Pitiriga, V. Serum β-Defensin 2, A Novel Biomarker for the Diagnosis of Acute Infections. Diagnostics 2023, 13, 1885. https://doi.org/10.3390/diagnostics13111885
Routsias JG, Marinou D, Mavrouli M, Tsakris A, Pitiriga V. Serum β-Defensin 2, A Novel Biomarker for the Diagnosis of Acute Infections. Diagnostics. 2023; 13(11):1885. https://doi.org/10.3390/diagnostics13111885
Chicago/Turabian StyleRoutsias, John G., Dionysia Marinou, Maria Mavrouli, Athanasios Tsakris, and Vassiliki Pitiriga. 2023. "Serum β-Defensin 2, A Novel Biomarker for the Diagnosis of Acute Infections" Diagnostics 13, no. 11: 1885. https://doi.org/10.3390/diagnostics13111885