A Systematic Review of Pharmacologic and Rehabilitative Treatment of Small Fiber Neuropathies
Abstract
:1. Introduction
Small Fiber Neuropathy
2. Methods
2.1. Search Strategy
2.2. Selection Criteria and Data Extraction
3. Results
3.1. Description of the Studies
3.2. Variations of Experimental Conditions across the Studies
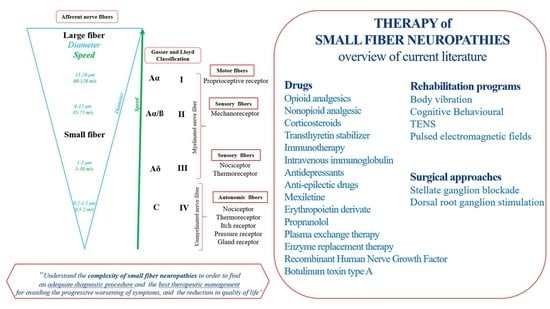
3.3. Pharmacologic and Rehabilitation Therapy
4. Discussion
4.1. Comparing Studies: Therapeutic Strategies
4.2. The Pharmacological Approaches
4.3. Surgical Approaches
4.4. Rehabilitative Program
4.5. Implication in Rehabilitation
5. Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bakkers, M.; Faber, C.G.; Hoeijmakes, J.G.J.; Lauria, G.; Merkies, I.S.J. Small Fibers, Large Impact: Quality of Life in Small-Fiber Neuropathy. Muscle Nerve 2014, 49, 329–336. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, 7647. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, 889–893. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.; Beecher, G.; Power, C.; Bridgland, L.; Zochodne, D.W. A Neuropathic Pain Syndrome Associated With Hantavirus Infection. J. Neurovirol. 2017, 23, 919–921. [Google Scholar] [CrossRef]
- Apfel, S.C.; Schwartz, S.; Adornato, B.T.; Freeman, R.; Biton, V.; Rendell, M.; Vinik, A.; Giuliani, M.; Stevens, J.C.; Barbano, R.; et al. Efficacy and Safety of Recombinant Human Nerve Growth Factor in Patients With Diabetic Polyneuropathy: A Randomized Controlled Trial. rhNGF Clinical Investigator Group. JAMA 2000, 284, 2215–2221. [Google Scholar] [CrossRef] [Green Version]
- Aradillas, E.; Schwartzman, R.J.; Grothusen, J.R.; Goebel, A.; Alexandder, G.M. Plasma Exchange Therapy in Patients with Complex Regional Pain Syndrome. Pain Physician 2015, 18, 383–394. [Google Scholar]
- Azmi, S.; Ferdousi, M.; Petropoulos, I.N.; Ponirakis, G.; Fadavi, H.; Tavakoli, M.; Alam, U.; Jones, W.; Marshall, A.; Jeziorska, M.; et al. Corneal Confocal Microscopy Shows an Improvement in Small-Fiber Neuropathy in Subjects With Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion Compared With Multiple Daily Injection. Diabetes Care 2015, 38, e3–e4. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, J.; Lalji, A.; Saed, A.; Baer, A.N. Biopsy-Proven Small-Fiber Neuropathy in Primary Sjögren’s Syndrome: Neuropathic Pain Characteristics, Autoantibody Findings, and Histopathologic Features. Arthritis Care Res. 2019, 71, 936–948. [Google Scholar] [CrossRef]
- Cao, T.; Yong, A.A.; Tan, K.B.; Tey, H.L. Idiopathic Aquagenic Pruritus: Pathogenesis and Effective Treatment with Atenolol. Dermatol. Ther. 2015, 28, 118–121. [Google Scholar] [CrossRef]
- Dabby, R.; Gilad, R.; Sadeh, M.; Lampl, Y.; Waterìmberg, N. Acute steroid responsive small-fiber sensory neuropathy: A new entity. J. Peripher. Nerv. Syst. 2006, 11, 47–52. [Google Scholar] [CrossRef]
- de Greef, B.; Merkies, I.S.J.; Geerts, M.; Faber, C.G.; Hoeijmakers, J.G.J. Efficacy, safety, and tolerability of lacosamide in patients with gain-of-function Nav1.7 mutation-related small fiber neuropathy: Study protocol of a randomized controlled trial-the LENSS study. Trials 2016, 17. [Google Scholar] [CrossRef] [Green Version]
- de Greef, B.T.; Geerts, M.; Hoeijmakers, J.G.; Faber, C.G.; Merkies, S.J. Intravenous immunoglobulin therapy for small fiber neuropathy: Study protocol for a randomized controlled trial. Trials 2016, 17, 330. [Google Scholar] [CrossRef] [Green Version]
- de Greef, B.T.; Hoeijmakers, J.G.; Geerts, M.; Oakes, M.; Church, T.J.; Waxman, S.G.; Dib-Hajj, S.D.; Faber, C.G.; Merkies, I.S. Lacosamide in patients with Nav1.7 mutations-related small fibre neuropathy: A randomized controlled trial. Brain 2019, 142, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Favon, V.; Liguoi, R.; Incensi, A.; Fileccia, E.; Donadio, V. The Incidental Finding of Elevated Anti GQ1B Antibodies in a Patient With Selective Small Fiber Neuropathy. J. Neurol. Sci. 2018, 388, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Gaillet, A.; Champion, K.; Lefaucheur, J.P.; Trout, H.; Bergmann, J.F.; Sene, D. Intravenous Immunoglobulin Efficacy for Primary Sjögren’s Syndrome Associated Small Fiber Neuropathy. Autoimmun. Rev. 2019, 18, 102387. [Google Scholar] [CrossRef]
- González-Duarte, A.; Lem, M.; Díaz-Díaz, E.; Castillo, C.; Cárdenas-Soto, K. The Efficacy of Pregabalin in the Treatment of Prediabetic Neuropathic Pain. Clin. J. Pain 2016, 32, 927–932. [Google Scholar] [CrossRef]
- Hilz, M.J.; Brys, M.; Marthol, H.; Stemper, B.; Dutsc, M. Enzyme Replacement Therapy for Fabry Disease. Neurology 2004, 62, 1066–1072. [Google Scholar] [CrossRef]
- Hoeijmakers, J.G.J.; Faber, C.G.; Miedema, C.J.; Merkies, I.S.J.; Vles, J.S.H. Small Fiber Neuropathy in Children: Two Case Reports Illustrating the Importance of Recognition. Pediatrics 2016, 138, e20161215. [Google Scholar] [CrossRef] [Green Version]
- Hoitsma, E.; Faber, C.G.; van Santen-Hoeufft, M.; De Vries, J.; Reulen, J.P.H.; Drent, M. Improvement of small fiber neuropathy in a sarcoidosis patient after treatment. Sarcoidosis Vasc. Diffuse Lung Dis. 2006, 23, 73–77. [Google Scholar]
- Hong, J.; Barnes, M.; Kessler, N. Case study: Use of vibration therapy in the treatment of diabetic peripheral small fiber neuropathy. J. Bodyw. Mov. Ther. 2013, 17, 235–238. [Google Scholar] [CrossRef]
- Kluding, P.M.; Pasnoor, M.; Singh, R.; Jernigan, S.; Farmer, K.; Rucker, J.; Sharma, N.K.; Wright, D.E. The Effect of Exercise on Neuropathic Symptoms, Nerve Function, and Cutaneous Innervation in People With Diabetic Peripheral Neuropathy. J. Diabetes Complicat. 2012, 26, 424–429. [Google Scholar] [CrossRef] [Green Version]
- Keohane, D.; Scwartz, J.; Gundapaneni, B.; Stewart, M.; Amass, L. Tafamidis delays disease progression in patients with early stage transthyretin familial amyloid polyneuropathy: Additional supportive analyses from the pivotal trial. Amyloid 2017, 24, 30–36. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Treister, R.; Lang, M.; Oaklander, A.L. IVIg for Apparently Autoimmune Small-Fiber Polyneuropathy: First Analysis of Efficacy and Safety. Ther. Adv. Neurol. Disord. 2018, 11, 1756285617744484. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, S.; Sharma, T.L.; Li, J.; Polston, D.; Li, Y. Longitudinal Follow-Up of Biopsy-Proven Small Fiber Neuropathy. Muscle Nerve 2019, 60, 376–381. [Google Scholar] [CrossRef]
- Maino, P.; Koetsier, E.; Kaelin-Lamg, A.; Gobbi, C.; Perez, R. Efficacious Dorsal Root Ganglion Stimulation for Painful Small Fiber Neuropathy: A Case Report. Pain Physician 2017, 20, E459–E463. [Google Scholar]
- Mishra, S.; Choudhary, P.; Joshi, S.; Bhatnagar, S. Successful Use of Flupirtine in Refractory Neuropathic Pain Due to Small Fiber Neuropathy. Am. J. Hosp. Palliat. Care 2013, 30, 91–93. [Google Scholar] [CrossRef]
- Morozumi, S.; Kawagashira, Y.; Lijima, M.; Koike, H.; Hattori, N.; Katsuno, M.; Tanaka, F.; Sobue, G. Intravenous Immunoglobulin Treatment for Painful Sensory Neuropathy Associated With Sjögren’s Syndrome. J. Neurol. Sci. 2009, 279, 57–61. [Google Scholar] [CrossRef]
- Namer, B.; Schmidt, D.; Eberhardt, E.; Maroni, M.; Dorfmeister, E.; Kleggetveit, I.P.; Kaluza, L.; Meents, J.; Gerlach, A.; Lin, Z.; et al. Pain Relief in a Neuropathy Patient by Lacosamide: Proof of Principle of Clinical Translation From Patient-Specific iPS Cell-Derived Nociceptors. EBioMedicine 2019, 39, 401–408. [Google Scholar] [CrossRef] [Green Version]
- Nevoret, M.; Vinik, A.I. CIDP Variants in Diabetes: Measuring Treatment Response with a Small Nerve Fiber Test. J. Diabetes Complicat. 2015, 29, 313–317. [Google Scholar] [CrossRef]
- Otis, J.D.; Sanderson, K.; Hardway, C.; Pincus, M.; Soumekh, S. A Randomized Controlled Pilot Study of a Cognitive-Behavioral therapy approach for painful diabetic peripheral neuropathy. J. Pain 2013, 14, 475–482. [Google Scholar] [CrossRef]
- Parambil, J.G.; Tavee, J.O.; Zhou, L.; Pearson, K.S.; Culver, D.A. Efficacy of intravenous immunoglobulin for small fiber neuropathy associated with sarcoidosis. Respir. Med. 2011, 105, 101–105. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.; Zhang, Y.; Unikel, L.H.; Edwards, C. A case of sporadic erythromelalgia presenting with small fibre neuropathy. BMJ Case Rep. 2019, 12, e230549. [Google Scholar] [CrossRef]
- Pereira, P.R.; Viala, K.; Maisonobe, T.; Haroche, J.; Mathian, A.; Hiè, M.; Amoura, Z.; Aubart, F.C. Sjögren Sensory Neuronopathy (Sjögren Ganglionopathy): Long-Term Outcome and Treatment Response in a Series of 13 Cases. Medicine 2016, 95, e3632. [Google Scholar] [CrossRef]
- Saito, H.; Yamaguchi, T.; Adachi, Y.; Yamashita, T.; Wakai, Y.; Saito, K.; Shinora, Y.; Suzuki, K.; Yagihasshi, S.; Terada, J.; et al. Neurological Symptoms of Sarcoidosis-induced Small Fiber Neuropathy Effectively Relieved With High-dose Steroid Pulse Therapy. Intern. Med. 2015, 54, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Schiffann, R.; Hauer, P.; Freeman, B.; Ries, M.; Scott, L.J.; Polydefkis, M.; Brady, R.O.; McArthur, J.C.; Wagner, K. Enzyme Replacement Therapy and Intraepidermal Innervation Density in Fabry Disease. Muscle Nerve 2006, 34, 53–56. [Google Scholar] [CrossRef]
- Smith, G.; Russell, J.; Feldman, E.L.; Goldstein, J.; Peltier, A.; Smith, S.; Hamwi, J.; Pollari, D.; Bixby, B.; Howard, J.; et al. Lifestyle Intervention for Pre-Diabetic Neuropathy. Diabetes Care 2006, 29, 1294–1299. [Google Scholar] [CrossRef] [Green Version]
- Tavee, J.O.; Karwa, K.; AAhmed, Z.; Thompson, N.; Parambil, J.; Culver, D.A. Sarcoidosis-associated Small Fiber Neuropathy in a Large Cohort: Clinical Aspects and Response to IVIG and anti-TNF Alpha Treatment. Respir. Med. 2017, 126, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Uyesugi, B.; Lippincott, B.; Dave, S. Treatment of a Painful Keloid With Botulinum Toxin Type, A. Am. J. Phys. Med. Rehabil. 2010, 89, 153–155. [Google Scholar] [CrossRef]
- Wakasugi, D.; Kato, T.; Gono, T.; Ito, E.; Nodera, H.; Kawaguchi, Y.; Yamanaka, H.; Hara, M. Extreme Efficacy of Intravenous Immunoglobulin Therapy for Severe Burning Pain in a Patient With Small Fiber Neuropathy Associated With Primary Sjögren’s Syndrome. Mod. Rheumatol. 2009, 19, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Waldinger, M.D.; Venema, P.L.; van Gils, A.P.; de Lint, G.J.; Schweitzer, D.H. Stronger evidence for small fiber sensory neuropathy in restless genital syndrome: Two case reports in males. J. Sex. Med. 2011, 8, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Walega, D.R.; Smith, C.; Epstein, J.B. Oral Pain From Burning Mouth Syndrome: A Case Report. J. Oral Facial Pain Headache Spring 2014, 28, 171–175. [Google Scholar] [CrossRef]
- Weintraub, M.I.; Herrmann, D.N.; Smith, A.G.; Backonja, M.M.; Cole, S.P. Pulsed Electromagnetic Fields to Reduce Diabetic Neuropathic Pain and Stimulate Neuronal Repair: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2009, 90, 1102–1109. [Google Scholar] [CrossRef]
- Windebank, A.J.; Sorenson, E.J.; Civil, R.; O’Brien, P.C. Role of Insulin-Like Growth factor-I in the Treatment of Painful Small Fiber Predominant Neuropathy. J. Peripher. Nerv. Syst. 2004, 9, 183–189. [Google Scholar] [CrossRef]
- Yuki, N.; Chan, A.C.; Wong, A.H.Y.; Inoue, T.; Yokai, M.; Kurihara, T.; Devaux, J.J.; Wider-Smith, E. Acute Painful Autoimmune Neuropathy: A Variant of Guillain-Barré Syndrome. Muscle Nerve 2018, 57, 320–324. [Google Scholar] [CrossRef]
- van Velzen, M.; Heij, L.; Niesters, M.; Cerami, A.; Dunne, A.; Dahan, A.; Brines, M. ARA 290 for Treatment of Small Fiber Neuropathy in Sarcoidosis. Expert Opin. Investig. Drugs 2014, 23, 541–550. [Google Scholar] [CrossRef]
- Oaklander, A.L. Immunotherapy Prospects for Painful Small-fiber Sensory Neuropathies and Ganglionopathies. Neurotherapeutics 2016, 13, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Kizawa, M.; Mori, K.; Iijima, M.; Koike, H.; Hattori, N.; Sobue, G. Intravenous immunoglobulin treatment in painful sensory neuropathy without sensory ataxia associated with Sjögren’s syndrome. J. Neurol. Neurosurg. Psychiatry 2006, 77, 967–969. [Google Scholar] [CrossRef] [Green Version]
- Chiaramonte, R.; Romano, M.; Vecchio, M. A Systematic Review of the Diagnostic Methods of Small Fiber Neuropathies in Rehabilitation. Diagnostics 2020, 10, 613. [Google Scholar] [CrossRef]
- Themistocleous, A.C.; Ramirez, J.D.; Serra, J.; Bennett, D.L.H. The Clinical Approach to Small Fibre Neuropathy and Painful Channelopathy. Pract. Neurol. 2014, 14, 368–379. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Li, J.; Ontaneda, D.; Sperling, J. Metabolic Syndrome in Small Fiber Sensory Neuropathy. J. Clin. Neuromuscul. Dis. 2011, 12, 235–243. [Google Scholar] [CrossRef]
- Lagerburg, V.; Bakkers, M.; Bouwhuis, A.; Hoeijmakers, J.G.J.; Smit, M.; Van Den Berg, S.J.M.; Hordijk-De Boer, I.; Brouwer-Van Der Lee, M.D.G.; Kranendonk, D.; Reulen, J.P.H.; et al. Contact Heat Evoked Potentials: Normal Values and Use in Small-Fiber Neuropathy. Muscle Nerve 2015, 51, 743–749. [Google Scholar] [CrossRef]
- Callaghan, B.C.; Little, A.A.; Feldman, E.L.; Hughes, R.A.C. Enhanced Glucose Control for Preventing and Treating Diabetic Neuropathy. Cochrane Database Syst. Rev. 2012, 6, CD007543. [Google Scholar] [CrossRef]
- Castelnuovo, G.; Giusti, E.M.; Manzoni, G.M.; Saviola, D.; Gatti, A.; Gabrielli, S.; Lacerenza, M.; Pietrabissa, G.; Cattivelli, R.; Spatola, C.A.; et al. Psychological Treatments and Psychotherapies in the Neurorehabilitation of Pain: Evidences and Recommendations from the Italian Consensus Conference on Pain in Neurorehabilitation. Front. Psychol. 2016, 7, 115. [Google Scholar] [CrossRef]
- Chan, A.C.Y.; Wilder-Smith, E.P.W. Small Fiber Neuropathy: Getting Bigger! Muscle Nerve 2016, 53, 671–682. [Google Scholar] [CrossRef]
- Tavee, J.O. Office Approach to Small Fiber Neuropathy. Cleve Clin. J. Med. 2018, 85, 801–812. [Google Scholar] [CrossRef]
- Attal, N.; Cruccu, G.; Baron, R.; Haanpaa, M.; Hanssom, P.; Jensen, T.S.; Nurmikko, T. EFNS Guidelines on the Pharmacological Treatment of Neuropathic Pain: 2010 Revision. Eur. J. Neurol. 2010, 17, 1113-e88. [Google Scholar] [CrossRef]
- Bril, V.; England, J.; Franklin, G.M.; Backonja, M.; Cohen, J.; Del Toro, D.; Feldman, R. Evidence-based Guideline: Treatment of Painful Diabetic Neuropathy: Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology 2011, 76, 1758–1765. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.J.; Jung, H.; Bhangoo, S.K.; White, F.A. Cytokine and Chemokine Regulation of Sensory Neuron Function. Handb. Exp. Pharmacol. 2009, 417–449. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Price, D.D.; Mayer, D.J. Mechanisms of Hyperalgesia and Morphine Tolerance: A Current View of Their Possible Interactions. Pain 1995, 62, 259–274. [Google Scholar] [CrossRef]
- Brouwer, B.; Merkies, I.J.; Gerrits, M.M.; Waxman, S.G.; Hoejmakers, J.G.J.; Faber, C.G. Painful Neuropathies: The Emerging Role of Sodium Channelopathies. J. Peripher. Nerv. Syst. 2014, 19, 53–65. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Iliadis, F.; Angelopoulou, N.; Perrea, D.; Ampatzidis, G.; Liapis, C.D.; Alevizos, M. The Anti-Inflammatory Effects of Exercise Training in Patients with Type 2 Diabetes Mellitus. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 837–843. [Google Scholar] [CrossRef]
- Maiorana, A.; O’Driscoll, G.; Cheetham, C.; Dembo, L.; Stanton, K.; Goodman, C.; Taylor, R.; Green, D. The Effect of Combined Aerobic and Resistance Exercise Training on Vascular Function in Type 2 Diabetes. J. Am. Coll. Cardiol. 2001, 38, 860–866. [Google Scholar] [CrossRef] [Green Version]

| Authors, Year | Study Design | Patients. Age | SFN Disease. Age at Onset Diagnosis | Onset SFN Symptoms | Symptoms | Therapy | Conclusions |
|---|---|---|---|---|---|---|---|
| Anderson 2017 [4] | Case report | 1 patient, 35 years old | SFN associated with hantavirus infection | One month after hantavirus infection | Severe, intractable burning limb pain. Allodynia to light touch and hyperalgesia to pinprick in a stocking distribution up to the mid-calf bilaterally | Gabapentin and naproxen | At follow-up 4 months later, his limb pain was only marginally improved |
| Apfel 2000 [5] | Clinical trial level 2 | A: 418 rhNGF B: 461 placebo 18–74 years | Diabetic SFN | - | Neuropathic pain | rhNGF | Significant beneficial effect of rhNGF on diabetic polyneuropathy |
| Aradillas 2015 [6] | Case series level 4 | 33 p, 45.7 years | SFN related to complex regional pain syndrome | 9.7 years | Neuropathic pain | Plasma exchange | Plasma exchange is effective for patients with severe long-standing complex regional pain syndrome |
| Azmi 2015 [7] | Observational study level 2 | 49 patients A: 18 patients with subcutaneous insulin infusion 55.4 ± 2.9 years B: 31 patients with daily insulin injection 49.9 ± 3.3 years | Diabetic SFN | A: 34.8 ± 3.1 years B: 35.2 ± 3.6 years | Neuropathic pain | Continuous subcutaneous insulin Infusion | Daily insulin injection group showed no significant change, but the subcutaneous insulin infusion group showed an improvement in corneal nerve morphology, consistent with regeneration |
| Birnbaum 2018 [8] | Observational study level 2 | 23 patients ~53.6 years 44 ± 13 years | Sjögren’s syndrome | 49.5 ± 23 years | Pain. Eleven patients had stocking-and-glove pain, and 12 patients had non-stocking and-glove pain. Ten SFN patients (~45%) had neuropathic pain preceding sicca symptoms. | Opioid analgesics were prescribed to ~45% of SFN patients | Sjögren’s syndrome SFN had increased frequency of male sex, decreased frequency of multiple antibodies, were frequently treated with opioid analgesics, and could present with nonstocking-and-glove pain |
| Cao 2015 [9] | Case report | 1 patient 36 years | SFN related to idiopathic aquagenic pruritus | ~for 3 y after symptoms | Aquagenic pruritus | Propranolol 10 mg bis in die for 1 month | Atenolol is to be preferred to propranolol, in view of its convenient once-a-day dosing and better side effect profile |
| Dabby 2006 [10] | Observational study level 2 | 4 patients ~49 years | Idiopathic SFN | - | Neuropathic pain. Symptoms were distal and symmetrical in three patients and generalized in one patient | Prednisone, 1 mg/kg for 12 weeks | Clinical improvement occurred 1–2 weeks after oral prednisone therapy was initiated. |
| De Greef 2016 [11] | Clinical trial level 2 | 25 patients 18–80 years | SCN9A-associated SFN | - | Pain, altered temperature sensation. | Lacosamide, 200 mg bis in die for 8 weeks | Lacosamide: a potential treatment option in patients with painful neuropathies, considering the central role of Nav1.7 in pain. |
| De Greef 2016 [12] | Clinical trial level 2 | 60 patients >18 years | Idiopathic SFN | - | Pain, altered temperature sensation. | Intravenous Immunoglobulins g/kg body weight over 2–4 consecutive days, followed by a maintenance dose of 1 g/kg body weight over 1–2 consecutive days given 3 times at a 3-weeks interval | Positive findings in SFN after intravenous immunoglobulins |
| De Greef 2019 [13] | Clinical trial level 2 | 24 patients ~48 years | SCN-SFN | - | Pain and autonomic dysfunction | Lacosamide, 200 mg bis in die for 8 weeks | Significant effect on pain, general wellbeing, and sleep quality |
| Favoni 2018 [14] | Case report | 1 patient 45 years | Anti-GQ1b antibodies associated with SFN | ~2 y after symptoms | Tingling and burning pain sensation in the arms and legs, with nocturnal exacerbation | Adalimumab: 40 mg every day, subcutaneous administration for 1 year | Benefit from immunotherapy |
| Gaillet 2019 [15] | Retrospective study level 2 | 11 patients 41–62 years | Sjögren’s syndrome | ~6.5 y after symptoms | Pain | 6 months intravenous immunoglobulins infusions, 0.4 g/kg/day for 5 days | Efficacy of intravenous immunoglobulins treatment for pain relief in Sjögren’s Syndrome-SFN with an improvement of quality of life and sensory testing |
| González-Duarte 2015 [16] | Clinical trial level 2 | 45 patients ~54 years | Prediabetic SFN | - | Neuropathic pain | Pregabalin was initiated at a dose of 75 mg and tapered up to 300 mg bis in die. | Improvement of prediabetic neuropathic pain with pregabalin |
| Hilz 2004 [17] | Observational study level 2 | 22 patients A: 11 patients B: 11 patients 27.9 ± 8 years C: 25 HC 29 ± 10.4 years | Fabry related SFN | - | Pain | Enzyme replacement therapy A: for 18 months B: for 23 months C: placebo Every 2 weeks, patients were treated with 0.9 to 1.1 mg/kg of agalsidase ß | Enzyme replacement therapy with agalsidase beta significantly improves function of C-, AΔ-, and Aß- nerve fibers and intradermal vibration receptors in Fabry neuropathy |
| Hoeijmakers 2016 [18] | Case reports | 2 patients ~15 years | 1 p idiopathic SFN, 1 p diabetic SFN | ~7 y after symptoms | Painful itch and tingling of legs, dysautonomia symptoms | Gabapentin | Moderate pain relief with treatment with gabapentin in a case. Treatment with duloxetine, combined with a rehabilitation program, resulted in a marked improvement in daily functioning. |
| Hoitsma 2006 [19] | Observational study level 2 | 1 patient 39 years | Sarcoidosis-associated SFN | - | fatigue, neuropathic pain, autonomic dysfunction, and arthralgia | Infliximab | SFN seems not an irreversible disorder; infliximab had good outcomes |
| Hong 2013 [20] | Case report | 1 patient 64 years | Diabetic SFN | ~2 years | Peripheral neuropathic pain in his both feet | Vibration therapy 3 min of vibration Treatment (total 12 min) at 20 Hz 5 times a week for 4 weeks | The whole-body vibration is a good complementary treatment |
| Kluding 2012 [21] | Observational study level 2 | 17 patients 58.4 ± 5.98 years | Diabetic SFN | 12.4 ± 12.2 years | Pain | 10 weeks aerobic and strengthening exercises | Exercises improve SFN symptoms |
| Keohane 2017 [22] | Clinical trial level 1 | A: 48 patients B: 44 patients 18–75 years | Amyloid SNF | - | Distal-to-proximal sensorimotor neuropathy with autonomic symptoms | A: Tafamidis, 20 mg/d for 18 months B: placebo | Tafamidis delays neurologic progression in early-stage ATTRV30M-FAP. |
| Liu 2018 [23] | Retrospective study level 2 | 55 patients 41 ± 17 years | Autoimmune SFN | 6.3 ± 6.3 years | Neuropathic pain | Intravenous immunoglobulins ≥1 g/kg/4 weeks for ≥3 months. | Intravenous immunoglobulins are safe and effective |
| MacDonald 2019 [24] | Retrospective study level 2 | 87 patients | SFN | 3.2 years | Neuropathic pain | Gabapentin (n = 69), pregabalin (n = 51), duloxetine (n = 41), tricyclic agents (n = 37), and topical cream (e.g., capsaicin, lidocaine; n = 29). | 45.5% of patients had, at some time, been treated with opioid medications for neuropathic pain. |
| Maino 2017 [25] | Case report | 1 patient 74 years | SFN | ~6 years after symptoms | Burning and shooting pain in feet | Dorsal Root Ganglion Stimulation | 20 months post-implantation, the patient continued to experience stimulation-induced paresthesia covering the entire pain area |
| Mishra 2012 [26] | Case report | 1 patient 22 years | SFN | ~6 months after symptoms | Neuropathic pain | Flupirtine 200 mg 3/d along with pregabalin 300 mg 2/d | Reduction of pain after flupirtine |
| Morozumi 2008 [27] | Observational study level 2 | 5 patients 61.8 years | Sarcoidosis-associated SFN | - | Neuropathic pain | Intravenous immunoglobulins 0.4 g/kg/d for 5 days | Beneficial after intravenous immunoglobulins therapy |
| Namer 2019 [28] | Case report | 1 patient 69 years | SNF | ~10 years after symptoms | Burning pain | Lacosamide 50 mg orally in the evening for 6 months | Lacosamide reduced pain in SFN |
| Nevoret 2014 [29] | Case report | 1 patient 60 years | Chronic inflammatory demyelinating polyneuropathy SNP | ~2 years | Neuropathic pain | Intravenous immunoglobulins: 6 doses total, 75 g each + Azathioprine 50 mg bis in die | Less burning, shooting pains and tingling |
| Otis 2013 [30] | Clinical trial level 2 | 20 patients 62.5 ± 10.9 year | Diabetic SFN | - | Neuropathic pain | A: 12 p cognitive-behavioral therapy B: 8 p traditional treatment | Cognitive-behavioral therapy reduced pain |
| Parambil 2010 [31] | Case series | 3 patients | Sarcoidosis-associated SFN | Intractable neuropathic pain, autonomic dysfunction | Intravenous immunoglobulins: 2-g/kg followed by 1-g/kg in 2-weeks, and then received maintenance doses of 1-g/kg every 4-weeks. | Intravenous immunoglobulins appear to be effective in relieving symptoms | |
| Patel 2019 [32] | Case report | 1 patient 31 years | SCN-SNF | ~10 years after symptoms | Erythromelalgia, painful flushing and burning paresthesia of the proximal extremities | Carbamazepine 200 mg bis in die | Carbamazepine reduced pain |
| Pereira 2016 [33] | Case series | 13 patients 55 years | Sjögren’s syndrome | ~3 years after symptoms | Neuropathic pain, Paresthesia | 7 p corticosteroids, 7 p mycophenolate mofetil, 6patients hydroxychloroquine, 5 patients intravenous immunoglobulins, 4 patients cyclophosphamide, 2 patients other immunosuppressive drugs | Treatment with corticosteroids with immunosuppressive drugs, as mycophenolate mofetil, had positive results. In contrast, intravenous immunoglobulins had disappointing results |
| Saito 2015 [34] | Case report | 1 patient 59 years | Sarcoidosis-associated SFN | 10 days | Progressive pain and hypoesthesia of the right lower back associated with fever and constipation | Methylprednisolone 1 g/d for 3 days, followed by prednisolone 40 mg/d | Neurological symptoms were effectively relieved with high-dose steroid therapy |
| Schiffmann 2006 [35] | Clinical trial level 2 | 25 patients ~34 years | Fabry disease-related SFN | - | Neuropathic pain | α galactosidase 0.2 mg/kg every 2 weeks followed for 12 months | Epidermal nerve fiber regeneration did not occur after enzyme replacement therapy |
| Smith 2006 [36] | Observational study level 2 | 32 patients 60 ± 8.4 years | Diabetic SFN | 7 ± 31 years | Neuropathic pain | Rehabilitative exercises | Rehabilitative exercises improved symptoms |
| Tavee 2016 [37] | Retrospective study level 2 |
| Sarcoidosis-associated SFN | 41 years | Pain, paresthesia, dysautonomic symptoms | Intravenous immunoglobulins 2 mg/kg bodyweight for 5 days; Anti-TNF alpha (infliximab) 5 mg/kg every 4 weeks | Beneficial from intravenous immunoglobulins and anti-TNF alpha in symptoms |
| Uyesugi 2010 [38] | Case report | 1 patient, 80 years | Keloid related SFN | 5 years after surgery | Itching, pain, and allodynia | Botulinum toxin type A, 100 U, diluted with 5 mL of preservative-free saline | A keloid was treated successfully with botulinum toxin type A. |
| Wakasugi 2009 [39] | Case report | 1 patient, 40 years | Sarcoidosis-associated SFN | 2 months | Paresthesia and burning pain in the distal upper and lower limbs. | Intravenous immunoglobulins 2 mg for 5 | Intravenous immunoglobulins therapy was immediately and extremely effective |
| Waldinger 2011 [40] | Case reports | 2 patients ~54.5 years | SFN | ~2.5 years | Unpleasant genital sensations of being on the edge of an orgasm, overactive bladder, absence of erection and ejaculation, or spontaneous ejaculations | TENS | In the male patient, the use of TENS clinically significantly reduced the symptoms of restless genital syndrome, in a female patient, TENS application had no effect on genital complaints and complaints of overactive bladder syndrome |
| Walega 2014 [41] | Case report | 1 patient 53 years | Burning mouth syndrome-related SFN | ~6.5 months | Bilateral burning pain in the anterior tongue and mucosa of the lips | Verbal rating scale, Patient’s Global Impression of Change | Bilateral stellate ganglion blockade |
| Weintraub 2009 [42] | Clinical trial level 1 | A: 90 patients 61.1 ± 10.4 B: 104 patients 60.6 ± 12.4 years | Diabetic SNF | - | Neuropathic pain | A: pulsed electromagnetic fields varying intensity and polarity 10–30 min session for 2 h maximum, daily, 12 w B: Sham group | Pulsed electromagnetic fields at this dosimetry were ineffective in reducing neuropathic pain |
| Windebank 2004 [43] | Clinical trial level 2 | A: 20 patients, 58.3 ± 12.2 years B: 20 patients 62.2 ± 10.7 years | SFN | >6 months | Painful, distal, symmetrical neuropathy | A: IGF-I, 0.05 mg/kg twice daily for 6 months B: placebo | IGF-I was safe but did not improve symptoms in this 6-month trial |
| Yuki 2018 [44] | Case report | 3 patients, ~27.3 years | SFN variant of Guillain–Barré syndrome | The three patients developed the symptoms 42, 6 and 11 d, respectively, after symptom onset | Pinprick sensation with hyperesthesia and brush allodynia in a glove-and-stocking distribution | 1 p oral prednisolone 40 Mg/d for 5 days 2 patients intravenous immunoglobulins | One patient showed no response to intravenous immunoglobulins but a good response to prednisolone. One patient had no significant improvement with prednisolone. One patient had a gradual spontaneous recovery |
| van Velzen 2014 [45] | Clinical trial level 2 | A: 12 patients B: 13 patients ~48.6 years | Sarcoidosis-associated SFN | 7 years between the current study and the diagnosis of sarcoidosis | Pain, allodynia, hyperalgesia | A: ARA290, an erythropoietin derivate, intravenous of 2 mg dissolved in 6 mL of normal saline, 3/weeks for 4 weeks B: placebo | Long-lasting beneficial effects of ARA 290 |
| Authors | Rehabilitation Program | Note |
|---|---|---|
| Hoeijmakes 2016 [18] | - | After no results with a pharmacologic approach, the rehabilitation program was added |
| Hong 2013 [20] | Four bouts of 3 min of vibration treatment (total 12 min) at 20 Hz five times a week for four weeks. | Body vibration reduced acute and long-term pain in diabetics |
| Kluding 2012 [21] | 10-week exercise program with both aerobic and strengthening and resistance exercises significantly improved selected measures of peripheral nerve function, with a reduction in pain and neuropathic symptoms | The rehabilitation improved the neuropathic symptoms, nerve function and cutaneous innervation Rehabilitative exercises were added to the pharmacologic approach in diabetic SFN |
| Otis 2013 [30] | Cognitive behavioral: each session of 60 min for 11 weeks | For neuropathic pain in diabetic neuropathy |
| Smith 2006 [36] | 80 min for 73 weeks with improvement in neuropathic symptoms | Rehabilitative program was added to the pharmacologic approach in diabetic SFN |
| Waldinger 2011 [40] | Transcutaneous electrical nerve stimulation (TENS) in SFN for restless genital syndrome (ReGS) of dorsal nerve penis and in overactive bladder syndrome (OAB). | - |
| Weintraub 2009 [42] | Pulsed electromagnetic fields was ineffective in reducing diabetic neuropathic pain | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vecchio, M.; Chiaramonte, R.; Romano, M.; Pavone, P.; Musumeci, G.; Mauro, G.L. A Systematic Review of Pharmacologic and Rehabilitative Treatment of Small Fiber Neuropathies. Diagnostics 2020, 10, 1022. https://doi.org/10.3390/diagnostics10121022
Vecchio M, Chiaramonte R, Romano M, Pavone P, Musumeci G, Mauro GL. A Systematic Review of Pharmacologic and Rehabilitative Treatment of Small Fiber Neuropathies. Diagnostics. 2020; 10(12):1022. https://doi.org/10.3390/diagnostics10121022
Chicago/Turabian StyleVecchio, Michele, Rita Chiaramonte, Marcello Romano, Piero Pavone, Giuseppe Musumeci, and Giulia Letizia Mauro. 2020. "A Systematic Review of Pharmacologic and Rehabilitative Treatment of Small Fiber Neuropathies" Diagnostics 10, no. 12: 1022. https://doi.org/10.3390/diagnostics10121022