Enhanced Colorimetric Signal for Accurate Signal Detection in Paper-Based Biosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Equipment
2.3. Stopped Colorimetric Signal in Solution
2.4. Unstopped Colorimetric Signal on Membrane
2.5. Stopped Colorimetric Signal on Membrane
2.6. Color Intensity Analysis
3. Results and Discussion
3.1. Stopped Colorimetric Signal in Solution
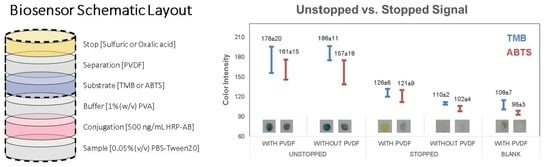
3.2. Unstopped Colorimetric Signal on Membrane
3.3. Stopped Colorimetric Signal on Membrane
3.4. Colorimetric Signal Stability Over Time
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harpaz, D.; Eltzov, E.; Seet, R.C.S.; Marks, R.S.; Tok, A.I.Y. Point-of-Care-Testing in Acute Stroke Management: An Unmet Need Ripe for Technological Harvest. Biosensors 2017, 7, 30. [Google Scholar] [CrossRef] [Green Version]
- Mahato, K.; Srivastava, A.; Chandra, P. Paper based diagnostics for personalized health care: Emerging technologies and commercial aspects. Biosens. Bioelectron. 2017, 96, 246–259. [Google Scholar] [CrossRef]
- Parolo, C.; Merkoçi, A. Paper-based nanobiosensors for diagnostics. Chem. Soc. Rev. 2013, 42, 450–457. [Google Scholar] [CrossRef]
- Piriya, V.S.A.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Chang, D.; Zakaria, S.; Deng, M.; Allen, N.; Tram, K.; Li, Y. Integrating Deoxyribozymes into Colorimetric Sensing Platforms. Sensors 2016, 16, 2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Wang, S.; Wang, L.; Li, F.; Pingguan-Murphy, B.; Lu, T.J.; Xu, F. Advances in paper-based point-of-care diagnostics. Biosens. Bioelectron. 2014, 54, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, D.; Eltzov, E.; Axelrod, T.; Marks, R.S.; Tok, A.I. Membrane Type Comparison and Modification to Modulate Sample Flow in Paper Diagnostics. Biochem. Eng. J. 2019, 155, 107483. [Google Scholar]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent Advances in Paper-Based Sensors. Sensors 2012, 12, 11505. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Xu, M.; Hou, L.; Chen, G.; Tang, D. Magnetic Bead-Based Reverse Colorimetric Immunoassay Strategy for Sensing Biomolecules. Anal. Chem. 2013, 85, 6945–6952. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.M.; Martins, V.C.; Prazeres, D.M.; Vojinovic, V.; Cabral, J.M.; Fonseca, L.P. Horseradish peroxidase: A valuable tool in biotechnology. Biotechnol. Annu. Rev. 2003, 9, 1387–2656. [Google Scholar]
- Van Weemen, B.; Schuurs, A.; Oostermeijer, M.; Raymakers, H.T. Immunoassay using antibody—Enzyme conjugates. Febs Lett. 1974, 43, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Verlander, C. Detection of horseradish peroxidase by colorimetry. Nonisot. DNA Probe Tech. 1992, 185–201. [Google Scholar]
- Song, Y.; Wei, W.; Qu, X. Colorimetric Biosensing Using Smart Materials. Adv. Mater. 2011, 23, 4215–4236. [Google Scholar] [CrossRef]
- Conyers, S.M.; Kidwell, D.A. Chromogenic substrates for horseradish peroxidase. Anal. Biochem. 1991, 192, 207–211. [Google Scholar] [CrossRef]
- Hosoda, H.; Takasaki, W.; Oe, T.; Tsukamoto, R.; Nambara, T. A Comparison of Chromogenic Substrates for Horseradish Peroxidase as a Label in Steroid Enzyme Immunoassay. Chem. Pharm. Bull. 1986, 34, 4177–4182. [Google Scholar] [CrossRef] [Green Version]
- Ngo, T.T.; Lenhoff, H.M. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal. Biochem. 1980, 105, 389–397. [Google Scholar] [CrossRef]
- Thiha, A.; Ibrahim, F. A colorimetric enzyme-linked immunosorbent assay (ELISA) detection platform for a point-of-care dengue detection system on a lab-on-compact-disc. Sensors 2015, 15, 11431–11441. [Google Scholar] [CrossRef] [Green Version]
- Mazzu-Nascimento, T.; Morbioli, G.G.; Milan, L.A.; Donofrio, F.C.; Mestriner, C.A.; Carrilho, E. Development and statistical assessment of a paper-based immunoassay for detection of tumor markers. Anal. Chim. Acta 2017, 950, 156–161. [Google Scholar] [CrossRef]
- Eltzov, E.; Marks, R.S. Colorimetric stack pad immunoassay for bacterial identification. Biosens. Bioelectron. 2017, 87, 572–578. [Google Scholar] [CrossRef]
- Eltzov, E.; Marks, R.S. Miniaturized Flow Stacked Immunoassay for Detecting Escherichia coli in a Single Step. Anal. Chem. 2016, 88, 6441–6449. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, D.; Axelrod, T.; Yitian, A.L.; Eltzov, E.; Marks, R.S.; Tok, A.I.Y. Dissolvable Polyvinyl-Alcohol Film, a Time-Barrier to Modulate Sample Flow in a 3D-Printed Holder for Capillary Flow Paper Diagnostics. Materials 2019, 12, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bio-Rad. PVDF Membrane.Copyright © 2018 Bio-Rad Laboratories, Inc. All Rights Reserved. 2018. Available online: http://www.bio-rad.com/en-st/sku/1620177-immun-blot-pvdf-membrane-roll-26-cm-x-3-3-m?ID=1620177 (accessed on 5 January 2020).
- Technologies, M.M.; Absorbent Pads. Copyright © 2018 Advanced Microdevices Pvt. Ltd. mdi All Rights Reserved. 2018. Available online: http://www.mdimembrane.com/absorbent-pads (accessed on 5 January 2020).
- Johannes, T.W.; Woodyer, R.D.; Zhao, H. High-throughput Screening Methods for Oxidoreductases. Protein Sci. Encycl. Online 2008, 77–93. [Google Scholar] [CrossRef]
- KPL. Comparison of ABTS, TMB, and OPD Peroxidase Substrate Systems. Technical Service Report. 2019. Available online: https://www.seracare.com/globalassets/seracare-resources/tg-comparison-of-abts-tmb-and-opd-peroxidase-substrate-systems.pdf (accessed on 5 January 2020).
- John Goka, A.K.; Farthing, M.J.G. The Use of 3, 3′, 5, 5′-Tetramethylbenzidine as a Peroxidase Substrate in Microplate Enzyme-Linked Immunosorbent Assay. J. Immunoass. 1987, 8, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Bos, E.S.; van der Doelen, A.A.; Rooy, N.V.; Schuurs, A.H.W.M. 3,3′,5,5′-Tetramethylbenzidine as an Ames Test Negative Chromogen for Horse-Radish Peroxidase in Enzyme-Immunoassay. J. Immunoass. 1981, 2, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Childs, R.E.; Bardsley, W.G. The steady-state kinetics of peroxidase with 2,2’-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. J. 1975, 145, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.J.; Dobson, A.D.W.; Field, J.A. Reduction of the 2,2’-Azinobis(3-ethylbenzthiazoline-6-sulfonate) cation radical by physiological organic acids in the absence and presence of manganese. Appl. Environ. Microbiol. 1998, 64, 2026–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Color Intensity | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| TMB | |||||||||
| HRP-Antibody Dilution | |||||||||
| 1:4K | 1:6K | 1:8K | 1:10K | 1:12K | 1:14K | 1:16K | 1:18K | ||
| TMB Volume | 10 µL | 146 ± 17 | 151 ± 14 | 145 ± 17 | 153 ± 10 | 137 ± 17 | 129 ± 15 | 139 ± 11 | 128 ± 12 |
| 20 µL | 176 ± 13 | 175 ± 7 | 172 ± 11 | 168 ± 13 | 168 ± 16 | 162 ± 7 | 161 ± 7 | 146 ± 8 | |
| 30 µL | 190 ± 13 | 183 ± 19 | 175 ± 16 | 178 ± 14 | 178 ± 8 | 163 ± 15 | 161 ± 12 | 148 ± 12 | |
| 40 µL | 195 ± 10 | 184 ± 13 | 184 ± 12 | 186 ± 19 | 178 ± 17 | 173 ± 7 | 170 ± 19 | 153 ± 20 | |
| ABTS | |||||||||
| HRP-Antibody Dilution | |||||||||
| 1:4K | 1:6K | 1:8K | 1:10K | 1:12K | 1:14K | 1:16K | 1:18K | ||
| ABTS Volume | 10 µL | 115 ± 13 | 95 ± 4 | 85 ± 6 | 101 ± 8 | 92 ± 5 | 75 ± 14 | 81 ± 15 | 62 ± 16 |
| 20 µL | 169 ± 9 | 143 ± 6 | 127 ± 13 | 129 ± 11 | 130 ± 13 | 99 ± 5 | 119 ± 6 | 97 ± 13 | |
| 30 µL | 180 ± 5 | 159 ± 13 | 149 ± 10 | 157 ± 17 | 154 ± 16 | 127 ± 9 | 138 ± 15 | 109 ± 13 | |
| 40 µL | 192 ± 8 | 172 ± 10 | 163 ± 9 | 171 ± 10 | 166 ± 14 | 147 ± 9 | 148 ± 15 | 122 ± 16 | |
| Color Intensity | ||||
|---|---|---|---|---|
| Time (min) | TMB | ABTS | ||
| Stopped | Unstopped | Stopped | Unstopped | |
| 0 | 38 ± 3 | 44 ± 6 | 35 ± 2 | 36 ± 2 |
| 8 | 67 ± 6 | 171 ± 14 | 77 ± 5 | 66 ± 7 |
| 20 | 79 ± 7 | 192 ± 18 | 81 ± 11 | 96 ± 12 |
| 30 | 80 ± 8 | 188 ± 22 | 89 ± 18 | 109 ± 14 |
| 40 | 77 ± 7 | 189 ± 18 | 96 ± 24 | 121 ± 12 |
| 50 | 77 ± 8 | 187 ± 17 | 102 ± 26 | 117 ± 15 |
| 60 | 81 ± 7 | 190 ± 15 | 115 ± 26 | 129 ± 14 |
| 90 | 83 ± 8 | 188 ± 13 | 122 ± 20 | 131 ± 13 |
| 100 | 86 ± 8 | 184 ± 20 | 130 ± 20 | 141 ± 15 |
| 110 | 88 ± 9 | 184 ± 18 | 117 ± 20 | 136 ± 13 |
| 120 | 92 ± 6 | 187 ± 14 | 126 ± 16 | 143 ± 13 |
| 140 | 89 ± 7 | 184 ± 16 | 129 ± 18 | 146 ± 14 |
| 150 | 92 ± 6 | 182 ± 17 | 123 ± 16 | 137 ± 12 |
| 180 | 96 ± 5 | 184 ± 15 | 134 ± 16 | 148 ± 11 |
| 210 | 103 ± 3 | 191 ± 12 | 136 ± 15 | 149 ± 9 |
| 240 | 99 ± 2 | 190 ± 10 | 135 ± 12 | 142 ± 8 |
| 300 | 111 ± 3 | 192 ± 11 | 152 ± 10 | 147 ± 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harpaz, D.; Eltzov, E.; Ng, T.S.E.; Marks, R.S.; Tok, A.I.Y. Enhanced Colorimetric Signal for Accurate Signal Detection in Paper-Based Biosensors. Diagnostics 2020, 10, 28. https://doi.org/10.3390/diagnostics10010028
Harpaz D, Eltzov E, Ng TSE, Marks RS, Tok AIY. Enhanced Colorimetric Signal for Accurate Signal Detection in Paper-Based Biosensors. Diagnostics. 2020; 10(1):28. https://doi.org/10.3390/diagnostics10010028
Chicago/Turabian StyleHarpaz, Dorin, Evgeni Eltzov, Timothy S. E. Ng, Robert S. Marks, and Alfred I. Y. Tok. 2020. "Enhanced Colorimetric Signal for Accurate Signal Detection in Paper-Based Biosensors" Diagnostics 10, no. 1: 28. https://doi.org/10.3390/diagnostics10010028