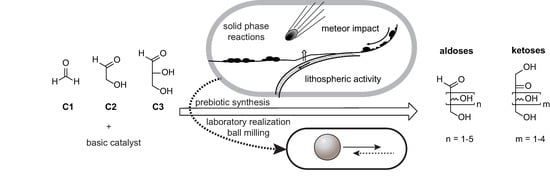
Prebiotic Sugar Formation Under Nonaqueous Conditions and Mechanochemical Acceleration
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Mineral-Catalyzed Mechanochemical Monosaccharide Synthesis
3.2. Model System: Nonaqueous Reaction
3.3. Model System: Kinetic Investigations of Mechanochemical Reaction
3.4. Comparison with Aqueous Reaction (C2+C2)
3.5. Built-In of Formaldehyde
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Butlerow, A. Bildung einer zuckerartigen Substanz durch Synthese. Justus Liebigs Ann. Chem. 1861, 120, 295–298. [Google Scholar] [CrossRef] [Green Version]
- Breslow, R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959, 1, 22–26. [Google Scholar] [CrossRef]
- Delidovich, I.V.; Simonov, A.N.; Taran, O.P.; Parmon, V.N. ChemInform Abstract: Catalytic Formation of Monosaccharides: From the Formose Reaction Towards Selective Synthesis. ChemInform 2014, 45. [Google Scholar] [CrossRef]
- Zafar, I.; Senad, N. The Formose Reaction: A Tool to Produce Synthetic Carbohydrates Within a Regenerative Life Support System. Curr. Org. Chem. 2012, 16, 769–788. [Google Scholar] [CrossRef] [Green Version]
- Socha, R.; Weiss, A.; Sakharov, M. Homogeneously catalyzed condensation of formaldehyde to carbohydrates: VII. An overall formose reaction model. J. Catal. 1981, 67, 207–217. [Google Scholar] [CrossRef]
- Tambawala, H.; Weiss, A.H. Homogeneously catalyzed formaldehyde condensation to carbohydrates: II. Instabilities and Cannizzaro effects. J. Catal. 1972, 26, 388–400. [Google Scholar] [CrossRef]
- Huskey, W.P.; Epstein, I.R. Autocatalysis and apparent bistability in the formose reaction. J. Am. Chem. Soc. 1989, 111, 3157–3163. [Google Scholar] [CrossRef]
- Simonov, A.N.; Pestunova, O.P.; Matvienko, L.G.; Parmon, V.N. The nature of autocatalysis in the Butlerov reaction. Kinet. Catal. 2007, 48, 245–254. [Google Scholar] [CrossRef]
- Evans, W.L. Some Less Familiar Aspects of Carbohydrate Chemistry. Chem. Rev. 1942, 31, 537–560. [Google Scholar] [CrossRef]
- El Khadem, H.S.; Ennifar, S.; Isbell, H.S. Evidence of stable hydrogen-bonded ions during isomerization of hexoses in alkali. Carbohydr. Res. 1989, 185, 51–59. [Google Scholar] [CrossRef]
- De Bruijn, J.M.; Kieboom, A.P.G.; Bekkiun, H.V. Alkaline Degradation of Monosaccharides VI1: The Fhucto-Fobmose Reaction of Mixtures of D-Fructose and Formaldehyde. J. Carbohydr. Chem. 1986, 5, 561–569. [Google Scholar] [CrossRef]
- Cleaves, H. Prebiotic Chemistry: Geochemical Context and Reaction Screening. Life 2013, 3, 331. [Google Scholar] [CrossRef]
- Darwin, C. Letter to J.D. Hooker; Hooker, J.D., Down, United Kingdom, 1871, published in: Darwin Correspondence Project, “Letter no. 7471”; Available online: http://www.darwinproject.ac.uk/DCP-LETT-7471 (accessed on 19 June 2019).
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Kitadai, N.; Nakamura, R.; Yamamoto, M.; Takai, K.; Li, Y.; Yamaguchi, A.; Gilbert, A.; Ueno, Y.; Yoshida, N.; Oono, Y. Geoelectrochemical CO production: Implications for the autotrophic origin of life. Sci. Adv. 2018, 4, eaao7265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, C.; Eisenreich, W.; Wächtershäuser, G. Synthesis of α-amino and α-hydroxy acids under volcanic conditions: Implications for the origin of life. Tetrahedron Lett. 2010, 51, 1069–1071. [Google Scholar] [CrossRef]
- Yamagata, Y.; Watanabe, H.; Saitoh, M.; Namba, T. Volcanic production of polyphosphates and its relevance to prebiotic evolution. Nature 1991, 352, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.P.; Miller, S.L. An efficient prebiotic synthesis of cytosine and uracil. Nature 1995, 375, 772–774. [Google Scholar] [CrossRef]
- Shapiro, R. Prebiotic cytosine synthesis: A critical analysis and implications for the origin of life. Proc. Natl. Acad. Sci. USA 1999, 96, 4396–4401. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, R. Comments on ‘concentration by evaporation and the prebiotic synthesis of cytosine’. Orig. Life Evol. Biosph. 2002, 32, 275–278. [Google Scholar] [CrossRef]
- Ross, D.S.; Deamer, D. Dry/Wet Cycling and the Thermodynamics and Kinetics of Prebiotic Polymer Synthesis. Life 2016, 6, 28. [Google Scholar] [CrossRef]
- Rode, B.M.; Fitz, D.; Jakschitz, T. The first steps of chemical evolution towards the origin of life. Chem. Biodivers. 2007, 4, 2674–2702. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L. How life began on Earth: A status report. Earth Planet. Sci. Lett. 2004, 226, 1–15. [Google Scholar] [CrossRef]
- Gull, M.; Zhou, M.; Fernandez, F.M.; Pasek, M.A. Prebiotic phosphate ester syntheses in a deep eutectic solvent. J. Mol. Evol. 2014, 78, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Menor-Salvan, C.; Marin-Yaseli, M.R. Prebiotic chemistry in eutectic solutions at the water-ice matrix. Chem. Soc. Rev. 2012, 41, 5404–5415. [Google Scholar] [CrossRef]
- Clark, B.; Kolb, V. Comet Pond II: Synergistic Intersection of Concentrated Extraterrestrial Materials and Planetary Environments to Form Procreative Darwinian Ponds. Life 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C. Primeval procreative comet pond. Orig. Life Evol. Biosph. 1988, 18, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Oberbeck, V.R.; Aggarwal, H. Comet impacts and chemical evolution on the bombarded Earth. Orig. Life Evol. Biosph. 1991, 21, 317–338. [Google Scholar] [CrossRef]
- Akouche, M.; Jaber, M.; Maurel, M.-C.; Lambert, J.-F.; Georgelin, T. Phosphoribosyl Pyrophosphate: A Molecular Vestige of the Origin of Life on Minerals. Angew. Chem. Int. Ed. 2017, 56, 7920–7923. [Google Scholar] [CrossRef]
- Coveney, P.V.; Swadling, J.B.; Wattis, J.A.; Greenwell, H.C. Theory, modelling and simulation in origins of life studies. Chem. Soc. Rev. 2012, 41, 5430–5446. [Google Scholar] [CrossRef]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef]
- Boldyreva, E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Mocci, R.; Schumacher, C.; Turberg, M.; Puccetti, F.; Hernández, J.G. Mechanochemical Activation of Iron Cyano Complexes: A Prebiotic Impact Scenario for the Synthesis of α-Amino Acid Derivatives. Angew. Chem. Int. Ed. 2018, 57, 2423–2426. [Google Scholar] [CrossRef] [PubMed]
- Bolm, C.; Hernández, J.G. From Synthesis of Amino Acids and Peptides to Enzymatic Catalysis: A Bottom-Up Approach in Mechanochemistry. ChemSusChem 2018, 11, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Eguaogie, O.; Vyle, J.S.; Conlon, P.F.; Gîlea, M.A.; Liang, Y. Mechanochemistry of nucleosides, nucleotides and related materials. Beilstein J. Org. Chem. 2018, 14, 955–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buczkowski, D.; Wyrick, D. Tectonism and Magmatism on Asteroids. In Proceedings of the European Planetary Science Congress, Nantes, France, 27 September–2 October 2015. [Google Scholar]
- Pallmann, S.; Šteflová, J.; Haas, M.; Lamour, S.; Henß, A.; Trapp, O. Schreibersite: An effective catalyst in the formose reaction network. New J. Phys. 2018, 20, 055003. [Google Scholar] [CrossRef]
- Belén, R.; Angelika, B.; Carsten, B. A Highly Efficient Asymmetric Organocatalytic Aldol Reaction in a Ball Mill. Chem. Eur. J. 2007, 13, 4710–4722. [Google Scholar]
- Heintz, A.S.; Gonzales, J.E.; Fink, M.J.; Mitchell, B.S. Catalyzed self-aldol reaction of valeraldehyde via a mechanochemical method. J. Mol. Catal. A Chem. 2009, 304, 117–120. [Google Scholar] [CrossRef]
- Haas, M.; Lamour, S.; Trapp, O. Development of an advanced derivatization protocol for the unambiguous identification of monosaccharides in complex mixtures by gas and liquid chromatography. J. Chromatogr. A 2018, 1568, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Willis, D.E.; Scanlon, J.T. Calculation of Flame Ionization Detector Relative Response Factors Using the Effective Carbon Number Concept. J. Chromatogr. Sci. 1985, 23, 333–340. [Google Scholar]
- Ritson, D.; Sutherland, J.D. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nat. Chem. 2012, 4, 895–899. [Google Scholar] [CrossRef] [Green Version]
- Cleaves Ii, H.J. The prebiotic geochemistry of formaldehyde. Precambrian Res. 2008, 164, 111–118. [Google Scholar] [CrossRef]
- Meinert, C.; Myrgorodska, I.; de Marcellus, P.; Buhse, T.; Nahon, L.; Hoffmann, S.V.; d’Hendecourt Lle, S.; Meierhenrich, U.J. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 2016, 352, 208–212. [Google Scholar] [CrossRef] [Green Version]
- de Marcellus, P.; Meinert, C.; Myrgorodska, I.; Nahon, L.; Buhse, T.; d’Hendecourt Lle, S.; Meierhenrich, U.J. Aldehydes and sugars from evolved precometary ice analogs: Importance of ices in astrochemical and prebiotic evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 965–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goesmann, F.; Rosenbauer, H.; Bredehoft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, T.; Giri, C.; Kruger, H.; Le Roy, L.; MacDermott, A.J.; et al. COMETARY SCIENCE. Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349, aab0689. [Google Scholar] [CrossRef] [PubMed]
- McCaffrey, V.P.; Zellner, N.E.; Waun, C.M.; Bennett, E.R.; Earl, E.K. Reactivity and survivability of glycolaldehyde in simulated meteorite impact experiments. Orig. Life Evol. Biosph. 2014, 44, 29–42. [Google Scholar] [CrossRef]
- Hazen, R.M. Paleomineralogy of the Hadean Eon: A preliminary species list. Am. J. Sci. 2013, 313, 807–843. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G.; Ingram, P.; Walker, G.L. Formose production by minerals: Possible relevance to the origin of life. J. Theor. Biol. 1972, 35, 601–604. [Google Scholar] [CrossRef]
- Schwartz, A.W.; de Graaf, R.M. The prebiotic synthesis of carbohydrates: A reassessment. J. Mol. Evol. 1993, 36, 101–106. [Google Scholar] [CrossRef]
- Chauhan, P.; Chimni, S.S. Mechanochemistry assisted asymmetric organocatalysis: A sustainable approach. Beilstein J. Org. Chem. 2012, 8, 2132–2141. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, B.; Rantanen, T.; Bolm, C. Solvent-Free Asymmetric Organocatalysis in a Ball Mill. Angew. Chem. 2006, 118, 7078–7080. [Google Scholar] [CrossRef]
- Kulla, H.; Haferkamp, S.; Akhmetova, I.; Röllig, M.; Maierhofer, C.; Rademann, K.; Emmerling, F. In Situ Investigations of Mechanochemical One-Pot Syntheses. Angew. Chem. Int. Ed. 2018, 57, 5930–5933. [Google Scholar] [CrossRef] [PubMed]
- Castells, J.; Geijo, F.; López-Calahorra, F. The “formoin reaction”: A promising entry to carbohydrates from formaldehyde. Tetrahedron Lett. 1980, 21, 4517–4520. [Google Scholar] [CrossRef]
- Eckhardt, A.K.; Linden, M.M.; Wende, R.C.; Bernhardt, B.; Schreiner, P.R. Gas-phase sugar formation using hydroxymethylene as the reactive formaldehyde isomer. Nat. Chem. 2018, 10, 1141–1147. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamour, S.; Pallmann, S.; Haas, M.; Trapp, O. Prebiotic Sugar Formation Under Nonaqueous Conditions and Mechanochemical Acceleration. Life 2019, 9, 52. https://doi.org/10.3390/life9020052
Lamour S, Pallmann S, Haas M, Trapp O. Prebiotic Sugar Formation Under Nonaqueous Conditions and Mechanochemical Acceleration. Life. 2019; 9(2):52. https://doi.org/10.3390/life9020052
Chicago/Turabian StyleLamour, Saskia, Sebastian Pallmann, Maren Haas, and Oliver Trapp. 2019. "Prebiotic Sugar Formation Under Nonaqueous Conditions and Mechanochemical Acceleration" Life 9, no. 2: 52. https://doi.org/10.3390/life9020052