Comparative Studies on the Anti-Inflammatory and Apoptotic Activities of Four Greek Essential Oils: Involvement in the Regulation of NF-κΒ and Steroid Receptor Signaling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Antibodies
2.4. Cell Viability Assay
2.5. Regulation of ERs, GR and NF-κB Transcriptional Activity via Essential Oils
2.6. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Essential Oils Effects on HEK-293 Cell Viability
3.2. Chios Mastic Essential Oil Caused Moderate Reduction in the E2-Induced Transcriptional Activation of ERα
3.3. Chios Mastic, Oregano, Melissa officinalis and Lavender Essential Oils Regulate the DEX-Induced Transcriptional Activation of GR
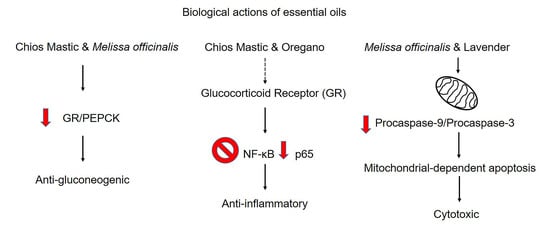
3.4. Effect of Essential Oils from Chios Mastic, Oregano, Melissa officinalis and Lavender on GR and PEPCK Protein Levels
3.5. Anti-Inflammatory Activities of Essential Oils from Chios Mastic, Oregano and Melissa officinalis via Suppression of NF-κB Transcriptional Activation
3.6. Regulation of the Mitochondrial-Dependent Apoptosis by the Essential Oils
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American type culture collection |
| DEX | Dexamethasone |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| E2 | Estradiol |
| EOs | Essential Oils |
| ER | Estrogen receptor |
| ERE | Estrogen Response Elements |
| ERα | Estrogen receptor alpha |
| ERβ | Estrogen receptor beta |
| FBS | Fetal Bovine Serum |
| G6Pase | Glucose 6-phosphatase |
| GR | Glucocorticoids Receptor |
| GREs -Luc | Glucocorticoid Response Elements-luciferase |
| HEK-293 | Human Embryonic Kidney-293 |
| HIF-1α | Hypoxia inducible factor 1 alpha |
| HPA | Hypothalamus-Pituitary-Adrenal |
| IL-6 | Interleukin-6 |
| NF-κB | Nuclear Factor Kappa B |
| NF-κB-REs-Luc | NF-κB Response Elements-luciferase |
| OD | Optical Density |
| PEPCK | Phoshoenolpyruvate carboxykinase |
| ROS | Reactive Oxygen Species |
| TNF-α | Tumor Necrosis factor alpha |
References
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Loza-Tavera, H. Monoterpenes in essential oils. Biosynthesis and properties. Adv. Exp. Med. Biol. 1999, 464, 49–62. [Google Scholar]
- Elshafie, H.S.; Camele, I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. BioMed Res. Int. 2017, 2017, 9268468. [Google Scholar] [CrossRef] [PubMed]
- Benny, A.; Thomas, J. Essential Oils as Treatment Strategy for Alzheimer’s Disease: Current and Future Perspectives. Planta Med. 2019, 85, 239–248. [Google Scholar] [PubMed] [Green Version]
- Barnes, P.J. Anti-inflammatory Actions of Glucocorticoids: Molecular Mechanisms. Clin. Sci. 1998, 94, 557–572. [Google Scholar] [CrossRef] [Green Version]
- Auphan, N.; DiDonato, J.A.; Rosette, C.; Helmberg, A.; Karin, M. Immunosuppression by glucocorticoids: Inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science 1995, 270, 286–290. [Google Scholar] [CrossRef]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [Green Version]
- Stice, J.P.; Knowlton, A.A. Estrogen, NFkappaB, and the heat shock response. Mol. Med. 2008, 14, 517–527. [Google Scholar] [CrossRef]
- Sundahl, N.; Bridelance, J.; Libert, C.; De Bosscher, K.; Beck, I.M. Selective glucocorticoid receptor modulation: New directions with non-steroidal scaffolds. Pharmacol. Ther. 2015, 152, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Cain, D.W.; Cidlowski, J.A. Specificity and sensitivity of glucocorticoid signaling in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 545–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgatza, D.; Gorgogietas, V.A.; Kylindri, P.; Charalambous, M.C.; Papadopoulou, K.K.; Hayes, J.M.; Psarra, A.-M.G. The triterpene echinocystic acid and its 3-O-glucoside derivative are revealed as potent and selective glucocorticoid receptor agonists. Int. J. Biochem. Cell Biol. 2016, 79, 277–287. [Google Scholar] [CrossRef] [PubMed]
- McColl, A.; Michlewska, S.; Dransfield, I.; Rossi, A.G. Effects of Glucocorticoids on Apoptosis and Clearance of Apoptotic Cells. Sci. World J. 2007, 7, 1165–1181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psarra, A.-M.G.; Sekeris, C.E. Nuclear receptors and other nuclear transcription factors in mitochondria: Regulatory molecules in a new environment. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2008, 1783, 1–11. [Google Scholar] [CrossRef] [Green Version]
- da Silva, J.S.; Montagnoli, T.L.; Rocha, B.S.; Tacco, M.L.C.A.; Marinho, S.C.P.; Zapata-Sudo, G. Estrogen Receptors: Therapeutic Perspectives for the Treatment of Cardiac Dysfunction after Myocardial Infarction. Int. J. Mol. Sci. 2021, 22, 525. [Google Scholar] [CrossRef]
- Gruver-Yates, A.L.; Cidlowski, J.A. Tissue-Specific Actions of Glucocorticoids on Apoptosis: A Double-Edged Sword. Cells 2013, 2, 202–223. [Google Scholar] [CrossRef] [Green Version]
- Paraschos, S.; Magiatis, P.; Gikas, E.; Smyrnioudis, I.; Skaltsounis, A.-L. Quality profile determination of Chios mastic gum essential oil and detection of adulteration in mastic oil products with the application of chiral and non-chiral GC–MS analysis. Fitoterapia 2016, 114, 12–17. [Google Scholar] [CrossRef]
- Vallianou, I.; Peroulis, N.; Pantazis, P.; Hadzopoulou-Cladaras, M. Camphene, a plant-derived monoterpene, reduces plasma cholesterol and triglycerides in hyperlipidemic rats independently of HMG-CoA reductase activity. PLoS ONE 2011, 6, e20516. [Google Scholar] [CrossRef] [Green Version]
- Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Lampri, E.; Fitsiou, E.; Vasileiadis, S.; Vamvakias, M.; Bardouki, H.; Goussia, A.; Malamou-Mitsi, V.; Panayiotidis, M.I.; et al. Dietary mastic oil extracted from Pistacia lentiscus var. chia suppresses tumor growth in experimental colon cancer models. Sci. Rep. 2017, 7, 3782. [Google Scholar] [CrossRef] [Green Version]
- Xanthis, V.; Fitsiou, E.; Voulgaridou, G.P.; Bogadakis, A.; Chlichlia, K.; Galanis, A.; Pappa, A. Antioxidant and Cytoprotective Potential of the Essential Oil Pistacia lentiscus var. chia and Its Major Components Myrcene and alpha-Pinene. Antioxidants 2021, 10, 127. [Google Scholar] [CrossRef]
- Georgiadis, I.; Karatzas, T.; Korou, L.-M.; Katsilambros, N.; Perrea, D. Beneficial Health Effects of Chios Gum Mastic and Peroxisome Proliferator-Activated Receptors: Indications of Common Mechanisms. J. Med. Food 2015, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Magkouta, S.; Stathopoulos, G.; Psallidas, I.; Papapetropoulos, A.; Kolisis, F.N.; Roussos, C.; Loutrari, H. Protective effects of mastic oil from Pistacia lentiscus variation chia against experimental growth of lewis lung carcinoma. Nutr. Cancer 2009, 61, 640–648. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bozin, B.; Sokovic, M.; Simin, N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef]
- de Sousa, A.C.; Alviano, D.S.; Blank, A.F.; Alves, P.B.; Alviano, C.S.; Gattass, C.R. Melissa officinalis L. essential oil: Antitumoral and antioxidant activities. J. Pharm. Pharmacol. 2004, 56, 677–681. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, N.C.; Corrêa-Angeloni, M.J.; Leffa, D.D.; Moreira, J.; Nicolau, V.; de Aguiar Amaral, P.; Rossatto, A.E.; de Andrade, V.M. Evaluation of the genotoxic and antigenotoxic potential of Melissa officinalis in mice. Genet. Mol. Biol. 2011, 34, 290–297. [Google Scholar] [CrossRef]
- Chung, M.J.; Cho, S.-Y.; Bhuiyan, M.J.H.; Kim, K.H.; Lee, S.-J. Anti-diabetic effects of lemon balm (Melissa officinalis) essential oil on glucose- and lipid-regulating enzymes in type 2 diabetic mice. Br. J. Nutr. 2010, 104, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Ayat, M.; Tameh, A.A.; Ghahremani, M.H.; Akbari, M.; Mehr, S.E.; Khanavi, M.; Hassanzadeh, G. Neuroprotective properties of Melissa officinalis after hypoxic-ischemic injury both in vitro and in vivo. DARU J. Pharm. Sci. 2012, 20, 42. [Google Scholar]
- Prusinowska, R.; Śmigielski, K.; Stobiecka, A.; Kunicka-Styczyńska, A. Hydrolates from lavender (Lavandula angustifolia)—Their chemical composition as well as aromatic, antimicrobial and antioxidant properties. Nat. Prod. Res. 2016, 30, 386–393. [Google Scholar] [CrossRef]
- Donelli, D.; Antonelli, M.; Bellinazzi, C.; Gensini, G.F.; Firenzuoli, F. Effects of lavender on anxiety: A systematic review and meta-analysis. Phytomedicine 2019, 65, 153099. [Google Scholar] [CrossRef]
- Samuelson, R.; Lobl, M.; Higgins, S.; Clarey, D.; Wysong, A. The Effects of Lavender Essential Oil on Wound Healing: A Review of the Current Evidence. J. Altern. Complement. Med. 2020, 26, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, R.; Wang, Y.; Qing, C.; Wang, W.; Yang, Y. In Vitro and In Vivo Efficacy Studies of Lavender angustifolia Essential Oil and Its Active Constituents on the Proliferation of Human Prostate Cancer. Integr. Cancer Ther. 2017, 16, 215–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Falco, E.; Mancini, E.; Roscigno, G.; Mignola, E.; Taglialatela-Scafati, O.; Senatore, F. Chemical Composition and Biological Activity of Essential Oils of Origanum vulgare L. subsp. vulgare L. under Different Growth Conditions. Molecules 2013, 18, 14948–14960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kountouri, A.M.; Gioxari, A.; Karvela, E.; Kaliora, A.C.; Karvelas, M.; Karathanos, V.T. Chemopreventive properties of raisins originating from Greece in colon cancer cells. Food Funct. 2013, 4, 366–372. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Fitsiou, E.; Bouloukosta, E.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Oreopoulou, A.; Papavassilopoulou, E.; Pappa, A.; Chlichlia, K. Extraction, Chemical Composition, and Anticancer Potential of Origanum onites L. Essential Oil. Molecules 2019, 24, 2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Garcia, I.; Silva-Espinoza, B.; Ortega-Ramirez, L.; Leyva, J.; Siddiqui, M.W.; Valenzuela, M.R.C.; Gonzalez-Aguilar, G.; Zavala, J.F.A. Oregano Essential Oil as an Antimicrobial and Antioxidant Additive in Food Products. Crit. Rev. Food Sci. Nutr. 2016, 56, 1717–1727. [Google Scholar] [CrossRef] [PubMed]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Misharina, T.A.; Burlakova, E.B.; Fatkullina, L.D.; Alinkina, E.S.; Vorob’eva, A.K.; Medvedeva, I.B.; Erokhin, V.N.; Semenov, V.A.; Nagler, L.G.; Kozachenko, A.I. Effect of oregano essential oil on the engraftment and development of Lewis carcinoma in F1 DBA C57 black hybrid mice. Prikl. Biokhim. Mikrobiol. 2013, 49, 432–436. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kalousi, F.D.; Pollastro, F.; Christodoulou, E.C.; Karra, A.G.; Tsialtas, I.; Georgantopoulos, A.; Salamone, S.; Psarra, A.G. Apoptotic, Anti-Inflammatory Activities and Interference with the Glucocorticoid Receptor Signaling of Fractions from Pistacia lentiscus L. var. chia Leaves. Plants 2022, 11, 934. [Google Scholar] [CrossRef] [PubMed]
- Tsialtas, I.; Georgantopoulos, A.; Karipidou, M.E.; Kalousi, F.D.; Karra, A.G.; Leonidas, D.D.; Psarra, A.-M.G. Anti-Apoptotic and Antioxidant Activities of the Mitochondrial Estrogen Receptor Beta in N2A Neuroblastoma Cells. Int. J. Mol. Sci. 2021, 22, 7620. [Google Scholar] [CrossRef]
- Miyamoto, T.; Okimoto, T.; Kuwano, M. Chemical Composition of the Essential Oil of Mastic Gum and their Antibacterial Activity Against Drug-Resistant Helicobacter pylori. Nat. Prod. Bioprospect. 2014, 4, 227–231. [Google Scholar] [CrossRef] [Green Version]
- Cassuto, H.; Kochan, K.; Chakravarty, K.; Cohen, H.; Blum, B.; Olswang, Y.; Hakimi, P.; Xu, C.; Massillon, D.; Hanson, R.W.; et al. Glucocorticoids Regulate Transcription of the Gene for Phosphoenolpyruvate Carboxykinase in the Liver via an Extended Glucocorticoid Regulatory Unit. J. Biol. Chem. 2005, 280, 33873–33884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oray, M.; Abu Samra, K.; Ebrahimiadib, N.; Meese, H.; Foster, C.S. Long-term side effects of glucocorticoids. Expert Opin. Drug Saf. 2016, 15, 457–465. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and Anti-Inflammatory Activities of Essential Oils: A Short Review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Sousa, V.I.; Parente, J.F.; Marques, J.F.; Forte, M.A.; Tavares, C.J. Microencapsulation of Essential Oils: A Review. Polymers 2022, 14, 1730. [Google Scholar] [CrossRef]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M. The Nuclear Receptor Superfamily: A Rosetta Stone for Physiology. Mol. Endocrinol. 2005, 19, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.V. Steroid hormone receptors. Curr. Top Pathol. 1991, 83, 365–431. [Google Scholar] [PubMed]
- Bai, C.; Schmidt, A.; Freedman, L.P.; Hasson, S.A.; Fogel, A.I.; Wang, C.; MacArthur, R.; Guha, R.; Heman-Ackah, S.; Martin, S.; et al. Steroid Hormone Receptors and Drug Discovery: Therapeutic Opportunities and Assay Designs. ASSAY Drug Dev. Technol. 2003, 1, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Quattrocelli, M.; Zelikovich, A.S.; Salamone, I.M.; Fischer, J.A.; McNally, E.M. Mechanisms and Clinical Applications of Glucocorticoid Steroids in Muscular Dystrophy. J. Neuromuscul. Dis. 2021, 8, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Valderrama, L.D.R.L. Effects of essential oils on central nervous system: Focus on mental health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, T.R.; Oliveira, M.T.; Lima, A.L.; Bezerra-Santos, C.R.; Piuvezam, M.R. Gamma-Terpinene Modulates Acute Inflammatory Response in Mice. Planta Med. 2015, 81, 1248–1254. [Google Scholar] [CrossRef] [PubMed]
- Bartoňková, I.; Dvořák, Z. Assessment of endocrine disruption potential of essential oils of culinary herbs and spices involving glucocorticoid, androgen and vitamin D receptors. Food Funct. 2018, 9, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Rodriguez, D.; Allred, K. A Systematic Review of Essential Oils and the Endocannabinoid System: A Connection Worthy of Further Exploration. Evid.-Based Complement. Altern. Med. 2020, 2020, 8035301. [Google Scholar] [CrossRef]
- Baschant, U.; Culemann, S.; Tuckermann, J. Molecular determinants of glucocorticoid actions in inflammatory joint diseases. Mol. Cell. Endocrinol. 2013, 380, 108–118. [Google Scholar] [CrossRef]
- Morrone, L.A.; Rombolà, L.; Pelle, C.; Corasaniti, M.T.; Zappettini, S.; Paudice, P.; Bonanno, G.; Bagetta, G. The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: Implication of monoterpene hydrocarbons. Pharmacol. Res. 2007, 55, 255–262. [Google Scholar] [CrossRef]
- Costa, C.A.R.d.A.; Kohn, D.O.; de Lima, V.M.; Gargano, A.C.; Flório, J.C.; Costa, M. The GABAergic system contributes to the anxiolytic-like effect of essential oil from Cymbopogon citratus (lemongrass). J. Ethnopharmacol. 2011, 137, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Atsumi, T.; Tonosaki, K. Smelling lavender and rosemary increases free radical scavenging activity and decreases cortisol level in saliva. Psychiatry Res. 2007, 150, 89–96. [Google Scholar] [CrossRef]
- Toda, M.; Morimoto, K. Effect of lavender aroma on salivary endocrinological stress markers. Arch. Oral Biol. 2008, 53, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Qadeer, S.; Emad, S.; Perveen, T.; Yousuf, S.; Sheikh, S.; Sarfaraz, Y.; Sadaf, S.; Haider, S. Role of ibuprofen and lavender oil to alter the stress induced psychological disorders: A comparative study. Pak. J. Pharm. Sci. 2018, 31, 1603–1608. [Google Scholar]
- Srivflai, J.; Khorana, N.; Waranuch, N.; Wisuitiprot, W.; Suphrom, N.; Suksamrarn, A.; Ingkaninan, K. Germacrene Analogs are Anti-androgenic on Androgen-dependent Cells. Nat. Prod. Commun. 2016, 11, 1225–1228. [Google Scholar] [PubMed]
- Luderer, U.; Dv, H.; N, L.; Ks, K.; Ca, B. Faculty Opinions recommendation of Prepubertal gynecomastia linked to lavender and tea tree oils. N. Engl. J. Med. 2007, 356, 479–485. [Google Scholar]
- Ramsey, J.T.; Li, Y.; Arao, Y.; Naidu, A.; A Coons, L.; Diaz, A.; Korach, K.S. Lavender Products Associated With Premature Thelarche and Prepubertal Gynecomastia: Case Reports and Endocrine-Disrupting Chemical Activities. J. Clin. Endocrinol. Metab. 2019, 104, 5393–5405. [Google Scholar] [CrossRef]
- Shinohara, K.; Doi, H.; Kumagai, C.; Sawano, E.; Tarumi, W. Effects of essential oil exposure on salivary estrogen concentration in perimenopausal women. Neuro Endocrinol. Lett. 2017, 37, 567–572. [Google Scholar]
- Zhang, X.; Peng, Y.; Wu, C. Chicken embryonic toxicity and potential in vitro estrogenic and mutagenic activity of carvacrol and thymol in low dose/concentration. Food Chem. Toxicol. 2021, 150, 112038. [Google Scholar] [CrossRef] [PubMed]
- Politano, V.T.; McGinty, D.; Lewis, E.M.; Hoberman, A.M.; Christian, M.S.; Diener, R.M.; Api, A.M. Uterotrophic Assay of Percutaneous Lavender Oil in Immature Female Rats. Int. J. Toxicol. 2013, 32, 123–129. [Google Scholar] [CrossRef] [Green Version]
- Simões, B.M.; Kohler, B.; Clarke, R.; Stringer, J.; Frazer, L.N.; Young, K.; Rautemaa-Richardson, R.; Zucchini, G.; Armstrong, A.; Howell, S.J. Estrogenicity of essential oils is not required to relieve symptoms of urogenital atrophy in breast cancer survivors. Ther. Adv. Med. Oncol. 2018, 10, 1758835918766189. [Google Scholar] [CrossRef] [Green Version]
- Yin, Q.-H.; Yan, F.-X.; Zu, X.-Y.; Wu, Y.-H.; Wu, X.-P.; Liao, M.-C.; Deng, S.-W.; Yin, L.-L.; Zhuang, Y.-Z. Anti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2. Cytotechnology 2012, 64, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Balusamy, S.R.; Perumalsamy, H.; Huq, A.; Balasubramanian, B. Anti-proliferative activity of Origanum vulgare inhibited lipogenesis and induced mitochondrial mediated apoptosis in human stomach cancer cell lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef] [PubMed]
- Gautam, N.; Mantha, A.K.; Mittal, S. Essential Oils and Their Constituents as Anticancer Agents: A Mechanistic View. BioMed Res. Int. 2014, 2014, 154106. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgantopoulos, A.; Vougioukas, A.; Kalousi, F.D.; Tsialtas, I.; Psarra, A.-M.G. Comparative Studies on the Anti-Inflammatory and Apoptotic Activities of Four Greek Essential Oils: Involvement in the Regulation of NF-κΒ and Steroid Receptor Signaling. Life 2023, 13, 1534. https://doi.org/10.3390/life13071534
Georgantopoulos A, Vougioukas A, Kalousi FD, Tsialtas I, Psarra A-MG. Comparative Studies on the Anti-Inflammatory and Apoptotic Activities of Four Greek Essential Oils: Involvement in the Regulation of NF-κΒ and Steroid Receptor Signaling. Life. 2023; 13(7):1534. https://doi.org/10.3390/life13071534
Chicago/Turabian StyleGeorgantopoulos, Achilleas, Athanasios Vougioukas, Foteini D. Kalousi, Ioannis Tsialtas, and Anna-Maria G. Psarra. 2023. "Comparative Studies on the Anti-Inflammatory and Apoptotic Activities of Four Greek Essential Oils: Involvement in the Regulation of NF-κΒ and Steroid Receptor Signaling" Life 13, no. 7: 1534. https://doi.org/10.3390/life13071534