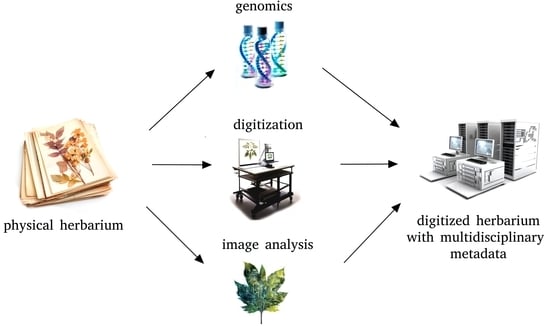
From Dormant Collections to Repositories for the Study of Habitat Changes: The Importance of Herbaria in Modern Life Sciences
Abstract
:1. From Figurative Herbaria to Phenological Studies
2. Herbaria and the DNA Sequencing Revolution
3. Botanical Collections in Italy and the Italian National Biodiversity Future Center
4. Future Challenges and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitman, N.C.; Jorgensen, P.M. Estimating the size of the world’s threatened Flora. Science 2002, 298, 986–987. [Google Scholar] [CrossRef] [PubMed]
- Travis, J.M.J. Climate change and habitat destruction: A deadly anthropogenic cocktail. Proc. R. Soc. Lond. Ser. B 2003, 270, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Rinawati, F.; Stein, K.; Lindner, A. Climate Change Impacts on Biodiversity. The setting of a lingering global crisis. Diversity 2013, 5, 114–123. [Google Scholar] [CrossRef]
- Palombo, M.R. Thinking about the biodiversity loss in this changing world. Geosciences 2021, 11, 370. [Google Scholar] [CrossRef]
- Pörtner, H.-O.; Roberts, D.C.; Tignor, M.; Poloczanska, E.S.; Mintenbeck, K.; Alegría, A.; Craig, M.; Langsdorf, S.; Löschke, S.; Möller, V.; et al. Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, MA, USA, 2022. [Google Scholar]
- Barrio, I.C.; Rapini, A. Plants under pressure: The impact of environmental change on plant ecology and evolution. BMC Ecol. Evol. 2023, 23, e13. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.S.; Carnaval, A.C.; Flantua, S.G.; Lohmann, L.G.; Ribas, C.C.; Riff, D. Human impacts outpace natural processes in the Amazon. Science 2023, 379, eabo5003. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.; Nimis, P.L.; Tretiach, M.; Muggia, L.; Moro, A.; Martellos, S. The Italian lichens dataset from the TSB herbarium (University of Trieste). Biodivers. Data J. 2023, 11, e96466. [Google Scholar] [CrossRef]
- Buldrini, F.; Pezzi, G.; Barbero, M.; Alessandrini, A.; Amadei, L.; Andreatta, S.; Ardenghi, N.M.G.; Armiraglio, S.; Bagella, S.; Bolpagni, R.; et al. The invasion history of Elodea canadensis and E. nuttallii (Hydrocharitaceae) in Italy from herbarium accessions, field records and historical literature. Biol. Invasions 2023, 25, 827–846. [Google Scholar] [CrossRef]
- Papalini, S.; Di Vittori, V.; Pieri, A.; Allegrezza, M.; Frascarelli, G.; Nanni, L.; Bitocchi, E.; Bellucci, E.; Gioia, T.; Pereira, L.G. Challenges and opportunities behind the use of herbaria in paleogenomics studies. Plants 2023, 12, 3452. [Google Scholar] [CrossRef]
- Willis, C.G.; Ellwood, E.R.; Primack, R.B.; Davis, C.C.; Pearson, K.D.; Gallinat, A.S.; Yost, J.M. Old plants, new tricks: Phenological research using herbarium specimens. Trends Ecol. Evol. 2017, 32, 531–546. [Google Scholar] [CrossRef]
- Nelson, G.; Sweeney, P.; Wallace, L.E.; Rabeler, R.K.; Allard, D.; Brown, H.; Carter, J. Digitization workflows for flat sheets and packets of plants, algae, and fungi. Appl. Plant Sci. 2015, 3, 1500065. [Google Scholar] [CrossRef]
- Harris, K.M.; Marsico, T.D. Digitizing specimens in a small herbarium: A viable workflow for collections working with limited resources. Appl. Plant Sci. 2017, 5, e1600125. [Google Scholar] [CrossRef]
- Heberling, J.M.; Prather, L.A.; Tonsor, S.J. The changing uses of herbarium data in an era of global change: An overview using automated content analysis. BioScience 2019, 69, 812–822. [Google Scholar] [CrossRef]
- Hedrick, B.; Heberling, M.; Meineke, E.; Turner, K.; Grassa, C.; Park, D.; Kennedy, J.; Clarke, J.; Cook, J.; Blackburn, D. Digitization and the future of natural history collections. Bioscience 2020, 70, 243–251. [Google Scholar] [CrossRef]
- Davis, W.J.; Crouch, J.A. Analysis of digitized herbarium records and community science observations provides a glimpse of downy mildew species diversity of North America, reveals potentially undescribed species, and documents the need for continued digitization and collecting. Fungal Ecol. 2022, 55, e101126. [Google Scholar] [CrossRef]
- Bellorini, C. The World of Plants in Renaissance Tuscany; Routledge: London, UK, 2016. [Google Scholar]
- Stefanaki, A.; Porck, H.; Grimaldi, I.N.; Thurn, N.; Pugliano, V.; Kardinaal, A.; Salemink, J. Breaking the silence of the 500-year-old smiling garden of everlasting flowers: The En Tibi book herbarium. PLoS ONE 2019, 14, e0217779. [Google Scholar] [CrossRef]
- Heberling, J.M. Herbaria as big data sources of plant traits. Int. J. Plant Sci. 2022, 183, 87–118. [Google Scholar] [CrossRef]
- Barnes, M.; Sulé-Suso, J.; Millett, J.; Roach, P. Fourier transform infrared spectroscopy as a non-destructive method for analysing herbarium specimens. Biol. Lett. 2023, 19, e20220546. [Google Scholar] [CrossRef]
- van der Ent, A.; Echevarria, G.; Pollard, A.J.; Erskine, P.D. X-ray fluorescence ionomics of herbarium collections. Sci. Rep. 2019, 9, e4746. [Google Scholar] [CrossRef]
- Azadnia, R.; Al-Amidi, M.M.; Mohammadi, H.; Cifci, M.A.; Daryab, A.; Cavallo, E. An AI based approach for medicinal plant identification using deep CNN based on global average pooling. Agronomy 2022, 12, 2723. [Google Scholar] [CrossRef]
- Weaver, W.N.; Smith, S.A. From leaves to labels: Building modular machine learning networks for rapid herbarium specimen analysis with LeafMachine2. Appl. Plant Sci. 2023, 11, e11548. [Google Scholar] [CrossRef] [PubMed]
- Gemeinholzer, B.; Dröge, G.; Zetzsche, H.; Haszprunar, G.; Klenk, H.; Güntsch, A.; Berendsohn, W.G.; Wägele, J. The DNA bank network: The start from a german initiative. Biopreserv. Biobank. 2011, 9, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Droege, G.; Barker, K.; Astrin, J.J.; Bartels, P.; Butler, C.; Cantrill, D.; Coddington, J.; Forest, F.; Gemeinholzer, B.; Hobern, D.; et al. The Global Genome Biodiversity Network (GGBN). Data Portal. Nucl. Acids Res. 2013, 42, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Mandrioli, M.; Borsatti, F.; Mola, L. Factors affecting DNA preservation from museum-collected lepidopteran specimens. Entomol. Exp. Appl. 2006, 120, 239–244. [Google Scholar] [CrossRef]
- Mandrioli, M. Insect collections and DNA analyses: How to manage collections? Mus. Manag. Curatorship 2008, 23, 193–199. [Google Scholar] [CrossRef]
- Holmes, M.W.; Hammond, T.T.; Wogan, G.O.U.; Walsh, R.E.; LaBarbera, K.; Wommack, E.A.; Martins, F.M.; Crawford, J.C.; Mack, K.L.; Bloch, L.M.; et al. Natural history collections as windows on evolutionary processes. Mol. Ecol. 2016, 25, 864–881. [Google Scholar] [CrossRef]
- Lopez, L.; Turner, K.G.; Bellis, E.S.; Lasky, J.R. Genomics of natural history collections for understanding evolution in the wild. Mol. Ecol. Resour. 2020, 20, 1153–1160. [Google Scholar] [CrossRef]
- Hart, M.L.; Forrest, L.L.; Nicholls, J.A.; Kidner, C.A. Retrieval of hundreds of nuclear loci from herbarium specimens. Taxon 2016, 65, 1081–1092. [Google Scholar] [CrossRef]
- Zedane, L.; Hong-Wa, C.; Murienne, J.; Jeziorski, C.; Baldwin, B.G.; Besnard, G. Museomics illuminate the history of an extinct, paleoendemic plant lineage (Hesperelaea, Oleaceae) known from an 1875 collection from Guadalupe Island, Mexico. Biol. J. Linn. Soc. 2016, 117, 44–57. [Google Scholar] [CrossRef]
- Särkinen, T.; Staats, M.; Richardson, J.E.; Cowan, R.S.; Bakker, F.T. How to open the treasure chest? Optimising DNA extraction from herbarium specimens. PLoS ONE 2012, 7, e43808. [Google Scholar] [CrossRef]
- Marinček, P.; Wagner, N.D.; Tomasello, S. Ancient DNA extraction methods for herbarium specimens: When is it worth the effort? Appl. Plant Sci. 2022, 10, e11477. [Google Scholar] [CrossRef] [PubMed]
- Staats, M.; Cuenca, A.; Richardson, J.E.; Vrielink-van Ginkel, R.; Petersen, G.; Seberg, O.; Bakker, F.T. DNA damage in plant herbarium tissue. PLoS ONE 2011, 6, e28448. [Google Scholar] [CrossRef]
- McNulty, S.N.; Mann, P.R.; Robinson, J.A.; Duncavage, E.J.; Pfeifer, J.D. Impact of reducing DNA input on Next-Generation sequencing library complexity and variant detection. J. Mol. Diagn. 2016, 22, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, L.D. A non-destructive DNA sampling technique for herbarium specimens. PLoS ONE 2017, 12, e0183555. [Google Scholar] [CrossRef] [PubMed]
- Sugita, N.; Ebihara, A.; Hosoya, T.; Jinbo, U.; Kaneko, S.; Kurosawa, T.; Nakae, M.; Yukawa, T. Non-destructive DNA extraction from herbarium specimens: A method particularly suitable for plants with small and fragile leaves. J. Plant Res. 2019, 133, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Burbano, H.A.; Gutaker, R.M. Ancient DNA genomics and the renaissance of herbaria. Science 2023, 382, 59–63. [Google Scholar] [CrossRef]
- Daru, B.H.; Davies, T.J.; Willis, C.G. Widespread homogenization of plant communities in the Anthropocene. Nat. Commun. 2021, 12, e6983. [Google Scholar] [CrossRef]
- Cuber, P.; Chooneea, D.; Geeves, G.; Salatino, S.; Creedy, T.J.; Griffin, C.; Sivess, L.; Barnes, I.; Price, B.; Misra, R. Comparing the accuracy and efficiency of third generation sequencing technologies, Oxford Nanopore Technologies, and Pacific Biosciences, for DNA barcode sequencing applications. Ecol. Genet. Genom. 2023, 28, e100181. [Google Scholar] [CrossRef]
- Moggi, G. Gli erbari in Italia dall’800 ad oggi. In 100 Anni di Ricerche Botaniche in Italia (1888–1988); Pedrotti, F., Ed.; Società Botanica Italiana: Firenze, Italy, 1988; Volume II, pp. 959–984. [Google Scholar]
- Moggi, G. Gli Erbari in Italia. In Il Grande Libro Degli Erbari Italiani; Taffetani, F., Ed.; Herbaria, Nardini Editore: Firenze, Italy, 2012; pp. 707–814. [Google Scholar]
- Ubrizsy Savoia, A. Le piante americane nell’Erbario di Ulisse Aldrovandi. Webbia 1993, XLVIII, 579–598. [Google Scholar]
- Ubrizsy Savoia, A. La biodiversità americana nell’opera di Aldrovandi. In L’erbario Dipinto di Ulisse Aldrovandi: Un Capolavoro del Rinascimento, a Cura di A; Maiarino, M., Minelli, A.L., Eds.; Monti e B. Negroni: Lecco, Italy, 1995; pp. 75–104. [Google Scholar]
- Buldrini, F.; Alessandrini, A.; Mossetti, U.; Muzzi, E.; Pezzi, G.; Soldano, A.; Nascimbene, J. Botanical memory: Five centuries of floristic changes revealed by a Renaissance herbarium (Ulisse Aldrovandi, 1551–1586). R. Soc. Open Sci. 2023, 10, e230866. [Google Scholar] [CrossRef]
- Roma-Marzio, F.; Peruzzi, L.; Bedini, G. Personal private herbaria: A valuable but neglected source of floristic data. The case of Italian collections today. Ital. Bot. 2017, 3, 7–15. [Google Scholar] [CrossRef]
- Lo Brutto, S.; Badalucco, A.; Iacovera, R.; Cilli, E.; Sarà, M. Checklist of the Mammal Collection Preserved at the University of Palermo under the Framework of the National Biodiversity Future Center. Diversity 2023, 15, 518. [Google Scholar] [CrossRef]
- Enrichetti, F.; Bavestrello, G.; Cappanera, V.; Mariotti, M.; Massa, F.; Merotto, L.; Povero, P.; Rigo, I.; Toma, M.; Tunesi, L.; et al. High Megabenthic Complexity and Vulnerability of a Mesophotic Rocky Shoal Support Its Inclusion in a Mediterranean MPA. Diversity 2023, 15, 933. [Google Scholar] [CrossRef]
- Mori, E.; Touloupakis, E.; Viviano, A.; Mazza, G. Opening a gate to shade some light: Alien land planarians in the Eastern Mediterranean and Northern Africa. Zootaxa 2023, 5319, 295–300. [Google Scholar] [CrossRef]
- Lozano, V.; Di Febbraro, M.; Brundu, G.; Carranza, M.L.; Alessandrini, A.; Ardenghi, N.M.G.; Barni, E.; Bedini, G.; Celesti-Grapow, L.; Cianfaglione, K.; et al. Plant invasion risk inside and outside protected areas: Propagule pressure, abiotic and biotic factors definitively matter. Sci. Total. Environ. 2023, 877, 162993. [Google Scholar] [CrossRef]
- Abbott, A. Hidden treasures: Florence’s botanical collection. Nature 2008, 452, e414. [Google Scholar] [CrossRef]
- Cuccuini, P. The types of Italian Flora in the Herbarium Centrale Italicum in relation to the original collections and their founders. Bocconea 2003, 16, 293–304. [Google Scholar]
- Contardi, S.; Cuccuini, P.; Nepi, C. Herbarium Centrale Italicum (the phanerogamic section): The genesis and structure of a Herbarium. Nuncius 2001, 16, 881–882. [Google Scholar]
- Sweeney, P.W.; Starly, B.; Morris, P.J.; Xu, Y.; Jones, A.; Radhakrishnan, S.; Grassa, C.J.; Davis, C.C. Large-scale digitization of herbarium specimens: Development and usage of an automated, high–throughput conveyor system. Taxon 2018, 67, 165–178. [Google Scholar] [CrossRef]
- Borsch, T.; Stevens, A.D.; Häffner, E.; Güntsch, A.; Berendsohn, W.G.; Appelhans, M.S.; Barilaro, C.; Beszter, B.; Blattner, F.R.; Bossdorf, O.; et al. A complete digitization of German herbaria is possible, sensible and should be started now. Res. Ideas Outcomes 2020, 6, e50675. [Google Scholar] [CrossRef]
- Thompson, K.M.; Birch, J.L. Mapping the digitisation workflow in a University herbarium. Res. Ideas Outcomes 2023, 9, e106883. [Google Scholar] [CrossRef]
- Wilkinson, M.; Dumontier, M.; Aalbersberg, I.J.; Appleton, G.; Axton, M.; Baak, A.; Blomberg, N.; Boiten, J.-W.; da Silva Santos, L.B.; Bourne, P.E.; et al. The FAIR Guiding Principles for scientific data management and stewardship. Sci. Data 2016, 3, 160018. [Google Scholar] [CrossRef] [PubMed]
- Albani Rocchetti, G.; Armstrong, C.G.; Abeli, T.; Orsenigo, S.; Jasper, C.; Joly, S.; Bruneau, A.; Zytaruk, M.; Vamosi, J.C. Reversing extinction trends: New uses of (old) herbarium specimens to accelerate conservation action on threatened species. New Phytol. 2021, 230, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Molano-Flores, B.; Johnson, S.A.; Marcum, P.B.; Feist, M.A. Utilizing herbarium specimens to assist with the listing of rare plants. Front. Conserv. Sci. 2023, 4, 1144593. [Google Scholar] [CrossRef]
- Meineke, E.K.; Classen, A.T.; Sanders, N.J.; Davies, J.T. Herbarium specimens reveal increasing herbivory over the past century. J. Ecol. 2019, 107, 105–117. [Google Scholar] [CrossRef]
- Guarino, S.; Basile, S.; Caimi, M.; Carratello, A.; Manachini, B.; Peri, E. Insect pests of the Herbarium of the Palermo botanical garden and evaluation of semiochemicals for the control of the key pest Lasioderma serricorne F. (Coleoptera: Anobiidae). J. Cult. Herit. 2020, 43, 37–44. [Google Scholar] [CrossRef]
- Beaulieu, C.; Lavoie, C.; Proulx, R. Bookkeeping of insect herbivory trends in herbarium specimens of purple loosestrife (Lythrum salicaria). Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 374, e20170398. [Google Scholar] [CrossRef]
- Marsico, T.; Krimmel, E.; Carter, J.R.; Gillespie, E.; Lowe, P.; McCauley, R.; Morris, A.; Nelson, G.; Smith, M.; Soteropoulos, D.; et al. Small herbaria contribute unique biogeographic records to county, locality, and temporal scales. Am. J. Bot. 2020, 107, 1577–1587. [Google Scholar] [CrossRef]
- Lang, P.L.M.; Willems, F.M.; Scheepens, J.F.; Burbano, H.A.; Bossdorf, O. Using herbaria to study global environmental change. New Phytol. 2019, 221, 110–122. [Google Scholar] [CrossRef]
- Muller, S. The use of herbarium specimens for investigating the spatio-temporal dynamics of biological invasions. Revue d’Ecologie 2015, 70, 229–235. [Google Scholar]
- Löbl, I.; Klausnitzer, B.; Hartmann, M.; Krell, F.-T. The silent extinction of species and taxonomists. An appeal to science policymakers and legislators. Diversity 2023, 15, 1053. [Google Scholar] [CrossRef]
- Roma-Marzio, F.; Maccioni, S.; Dolci, D.; Astuti, G.; Magrini, N.; Pierotti, F.; Vangelisti, R.; Amadei, L.; Peruzzi, L. Digitization of the historical Herbarium of Michele Guadagno at Pisa (PI-GUAD). PhytoKeys 2023, 234, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Ellis, S. The history and impact of digitization and digital data mobilization on biodiversity research. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 374, 20170391. [Google Scholar] [CrossRef] [PubMed]
- Lien, A.M.; Banki, O.; Bark, S.K.; Buckeridge, J.S.; Christidis, L.; Cigliano, M.M.; Conix, S.; Costello, M.J.; Hobern, D.; Kirk, P.M.; et al. Widespread support for a global species list with a formal governance system. Proc. Natl. Acad. Sci. USA 2023, 120, e2306899120. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandrioli, M. From Dormant Collections to Repositories for the Study of Habitat Changes: The Importance of Herbaria in Modern Life Sciences. Life 2023, 13, 2310. https://doi.org/10.3390/life13122310
Mandrioli M. From Dormant Collections to Repositories for the Study of Habitat Changes: The Importance of Herbaria in Modern Life Sciences. Life. 2023; 13(12):2310. https://doi.org/10.3390/life13122310
Chicago/Turabian StyleMandrioli, Mauro. 2023. "From Dormant Collections to Repositories for the Study of Habitat Changes: The Importance of Herbaria in Modern Life Sciences" Life 13, no. 12: 2310. https://doi.org/10.3390/life13122310