Citric Acid Changes the Fingerprint of Flavonoids and Promotes Their Accumulation in Phellinus igniarius (L.) Quél
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Conditions
2.2. Determination of Mycelial Biomass
2.3. Determination of Flavonoid Content
2.4. Experimental Parameters of Surface Response Method
2.5. Sample Preparations
2.6. Chromatographic Conditions
2.7. Statistical Analysis
3. Results
3.1. Effects of Different Concentrations of Citric Acid (CA) on Mycelial Biomass and Flavonoid Content of P. igniarius
3.2. Effect of CA Treatment at Different Times on Mycelial Biomass and Flavonoid Content of P. igniarius
3.3. Surface Response Method to Optimize CA Treatment Conditions
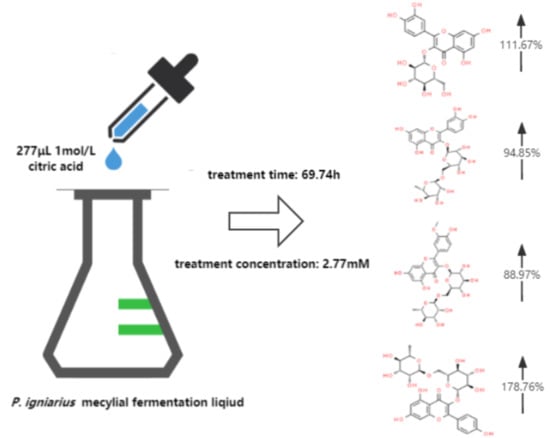
3.4. Effects of CA Treatment on Flavonoid Fingerprint of P. igniarius Mycelia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, N.C.; Wu, C.C.; Liu, R.H.; Chai, Y.C.; Tseng, C.Y. Comparing the functional components, SOD-like activities, antimutagenicity, and nutrient compositions of Phellinus igniarius and Phellinus linteus. J. Food Drug Anal. 2016, 24, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, S.K.; Batbayar, S.; Lee, D.H.; Kim, H.W. Activation of NADPH Oxidase by beta-Glucan from Phellinus baumii (Agaricomycetes) in RAW 264.7 Cells. Int. J. Med. Mushrooms 2017, 19, 957–965. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; He, Y.; Yu, Z.M.; Zhang, Y.; Wang, N.N.; Shou, D.; Li, C.Y. Metabolomic investigation of rat serum following oral administration of the willow bracket medicinal mushroom, phellinus igniarius (Agaricomycetes), by UPLC-HDMS. Int. J. Med. Mushrooms 2016, 18, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, F.; Yu, L.J.; Ji, J.X.; Wang, Y.Z. Flavonoid Accumulation in Cell Suspension Cultures of Glycyrrhiza inflata Batal under Optimizing Conditions. Z. Für Nat. C 2009, 64, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Collins, L.; Zhu, T.; Guo, J.; Xiao, Z.J.; Chen, C.Y. Phellinus linteus sensitises apoptosis induced by doxorubicin in prostate cancer. Br. J. Cancer 2006, 95, 282. [Google Scholar] [CrossRef]
- Hwang, B.S.; Lee, I.K.; Choi, H.J.; Yun, B.S. Anti-influenza activities of polyphenols from the medicinal mushroom Phellinus baumii. Bioorganic Med. Chem. Lett. 2015, 25, 3256–3260. [Google Scholar] [CrossRef]
- Wang, Y.C.; Yuan, Z.L.; Tan, X.R.; Ran, Z.H.; Jin, H. Optimization of Medium Components for the Production of Flavonoids and Soluble Protein with Phellinus Igniarius in Liquid Culture. In Proceedings of the 3rd International Conference on Applied Biotechnology (ICAB), Tianjin, China, 25–27 November 2016; pp. 421–431. [Google Scholar]
- Zhu, H.; Sun, S.J.; Zhang, S.S. Enhanced production of total flavones and exopolysaccharides via Vitreoscilla hemoglobin biosynthesis in Phellinus igniarius. Bioresour. Technol. 2011, 102, 1747–1751. [Google Scholar] [CrossRef]
- Zhang, B.B.; Cheung, P.C. Use of stimulatory agents to enhance the production of bioactive exopolysaccharide from Pleurotus tuberregium by submerged fermentation. J. Agric. Food Chem. 2011, 59, 1210–1216. [Google Scholar] [CrossRef]
- Ren, A.; Li, X.B.; Miao, Z.G.; Shi, L.; Jaing, A.L.; Zhao, M.W. Transcript and metabolite alterations increase ganoderic acid content in Ganoderma lucidum using acetic acid as an inducer. Biotechnol. Lett. 2014, 36, 2529–2536. [Google Scholar] [CrossRef]
- Shi, L.; Tan, Y.; Sun, Z.; Ren, A.; Zhao, M.W. Exogenous salicylic acid (SA) promotes the accumulation of biomass and flavonoid content in Phellinus igniarius (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 955–963. [Google Scholar] [CrossRef]
- Wang, J. Effect of Environmental Factors on Citrinin Production by Monascus; Tianjin University of Science of Technology: Tianjin, China, 2010; Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201401&filename=1013370405.nh&uniplatform=NZKPT&v=bL8gLjoBTbUs5svZeyhX0gUzKC4O8xRjzbizLaIsv6PG9I0H53jWrOPWzOcYktdL (accessed on 8 November 2022).
- Mingd, L.I.; Rui, Z.; Xiaolei, J.; Jun, Y.; Changxin, Z. Effects of adding intermediate material in tricarboxylic acid cycle on the activity of key enzymes of Saccharomyces cerevisiae TCA. Microbiol. China 2010, 37, 331–335. [Google Scholar]
- Ge, Y.H.; Wang, Y.; Bi, Y. Effect of Citric Acid Treatment on Inhibition of Muskmelon Black Spot and Phenylpropane Metabolism. Sci. Technol. Food Ind. 2013, 34, 308–312. [Google Scholar]
- Park, H.L.; Yoo, Y.; Bhoo, S.H.; Lee, T.H.; Cho, M.H. Two chalcone synthase isozymes participate redundantly in UV-Induced sakuranetin synthesis in rice. Int. J. Mol. Sci. 2020, 21, 3777. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.H.; Dai, D.K.; Zheng, B.Z.Y.; Duan, R.; Chan, G.K.L.; Dong, T.T.X.; Qin, Q.W.; Tsim, K.W.K. The binding of kaempferol-3-O-rutinoside to vascular endothelial growth factor potentiates anti-inflammatory efficiencies in lipopolysaccharide-treated mouse macrophage RAW264.7 cells. Phytomedicine 2021, 80, 153400. [Google Scholar] [CrossRef] [PubMed]
- Zhen, H.S.; He, L.Y.; Lu, S.Y.; Zhu, X.Y.; Mo, H.H.; Zhou, W.P.; Chen, J. Overview of fingerprinting studies on Chinese medicines. J. Guangxi Coll. Tradit. Chin. Med. 2005, 6, 63–65. [Google Scholar]
- Ding, S.; Dudley, E.; Plummer, S.; Tang, J.; Newton, R.P.; Brenton, A.G. Fingerprint profile of Ginkgo biloba nutritional supplements by LC/ESI-MS/MS. Phytochemistry 2008, 69, 1555–1564. [Google Scholar] [CrossRef]
- Acharya, B.; Assmann, S. Hormone interactions in stomatal function. Plant Mol. Biol. 2009, 69, 451–462. [Google Scholar] [CrossRef]
- Zapora, E.; Wolkowycki, M.; Bakier, S.; Zjawiony, J.K. Phellinus igniarius: A Pharmacologically Active Polypore Mushroom. Nat. Prod. Commun. 2016, 11, 1043–1046. [Google Scholar] [CrossRef] [Green Version]
- Weise, N.J.; Ahmed, S.T.; Parmeggiani, F.; Galman, J.L.; Dunstan, M.S.; Charnock, S.J.; Leys, D.; Turner, N.J. Zymophore identification enables the discovery of novel phenylalanine ammonia lyase enzymes. Sci. Rep. 2017, 7, 13691. [Google Scholar] [CrossRef] [Green Version]
- Dinkins, R.D.; Hancock, J.; Coe, B.L.; May, J.B.; Goodman, J.P.; Bass, W.T.; Liu, J.G.; Fan, Y.L.; Zheng, Q.L.; Zhu, H.Y. Isoflavone levels, nodulation and gene expression profiles of a CRISPR/Cas9 deletion mutant in the isoflavone synthase gene of red clover. Plant Cell Rep. 2021, 40, 517–528. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.H.; Qin, J.M. Simultaneous determination of seven flavonoids in the genus Qianjian by HPLC. Chin. Wild Plant Resour. 2011, 5, 54–57. [Google Scholar]
- Zhao, Z.N.; Yan, L.W.; Zhao, K. Fingerprinting of different origins and grades of Dioscorea. West China J. Pharm. 2020, 5, 518–522. [Google Scholar]
- Li, G.W.; Sun, D.M.; He, M.Y.; Wu, S.Z.; Wu, W.P.; Cao, S.Q. Study on HPLC Fingerprint and Determination of Flavonoids in Corolla cristata. Guid. J. Tradit. Chin. Med. Pharm. 2020, 13, 64–67. [Google Scholar]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jauhri, K.S.; Sen, A. Sen Production of protein by fungi from agricultural wastes: V. Effect of various organic acids and growth promoters on the efficiency of substrate utilization and protein production by Rhizoctonia melongina, Pleurotus ostreatus, and Coprinus aratus. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Zweite Nat. Abt. Mikrobiol. Der Landwirtsch. Der Technol. Und Des Umweltschutzes 1978, 133, 614–618. [Google Scholar]
- Liu, Y.T. The Effect of Citric Acid Treatment on the Chemical Composition and Biological Activity of Red Ginseng; Jilin Agricultural University: Changchun, China, 2018; Available online: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201801&filename=1017842630.nh&uniplatform=NZKPT&v=ockH47tlwbWQiBhwHlax3erY0DZWiCv88EJvdlLqQmDC97p8jJ07ue9ek4soZ6yC (accessed on 8 November 2022).
- Zhu, H.X.; Sun, J.X.; Li, Y.Y.; Li, X.Z. Growth factors increase the production of Phellinus igniarius mycelium and polysaccharides. Mod. Rural. Sci. Technol. 2019, 11, 63–64. [Google Scholar]
- Huang, C.; Wu, H.; Liu, Z.J.; Cai, J.; Lou, W.Y.; Zong, M.H. Effect of organic acids on the growth and lipid accumulation of oleaginous yeast Trichosporon fermentans. Biotechnol. Biofuels 2012, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Valentova, K.; Vrba, J.; Bancirova, M.; Ulrichova, J.; Kren, V. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef]
- Liu, T.T.; Cao, L.X.; Zhang, T.T.; Fu, H. Molecular docking studies, anti-Alzheimer’s disease, antidiabetic, and anti-acute myeloid leukemia potentials of narcissoside. Arch. Physiol. Biochem. 2020, 1–11. [Google Scholar] [CrossRef]
- Dubey, K.; Dubey, R. Computation screening of narcissoside a glycosyloxyflavone for potential novel coronavirus 2019 (COVID-19) inhibitor. Biomed. J. 2020, 43, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Shyaula, S.L.; Abbas, G.; Siddiqui, H.; Sattar, S.A.; Choudhary, M.I.; Basha, F.Z. Synthesis and Antiglycation Activity of Kaempferol-3-O-rutinoside (Nicotiflorin). Med. Chem. 2012, 8, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Gilani, A.U.H.; Aftab, K.; Ahmad, V.U. Effects of kaempferol-3-O-rutinoside on rat blood pressure. Phytother. Res. 1993, 7, 314–316. [Google Scholar] [CrossRef]
- Xie, Z.H.; Zhao, Y.; Chen, P.; Jing, P.; Yue, J.; Yu, L.L. Chromatographic Fingerprint Analysis and Rutin and Quercetin Compositions in the Leaf and Whole-Plant Samples of Di- and Tetraploid Gynostemma pentaphyllum. J. Agric. Food Chem. 2011, 59, 3042–3049. [Google Scholar] [CrossRef]
- Wang, C.C.; Chen, L.H.; Cai, Z.C.; Chen, C.X.; Liu, Z.X.; Liu, X.H.; Zou, L.S.; Chen, J.L.; Tan, M.X.; Wei, L.F.; et al. Dynamic Variations in Multiple Bioactive Constituents under Salt Stress Provide Insight into Quality Formation of Licorice. Molecules 2019, 24, 3670. [Google Scholar] [CrossRef]









| Run | Factor 1 | Factor 2 | Response 1 |
|---|---|---|---|
| A: Time (h) | B: Concentration (mM) | Flavonoid (mg/g) | |
| 1 | 48.00 | 0.00 | 56.65 ± 3.76 |
| 2 | 14.00 | 3.41 | 54.45 ± 1.19 |
| 3 | 48.00 | 4.00 | 55.89 ± 2.31 |
| 4 | 48.00 | 0.00 | 56.98 ± 4.54 |
| 5 | 0.00 | 2.00 | 56.21 ± 1.48 |
| 6 | 14.00 | 0.59 | 55.17 ± 1.99 |
| 7 | 82.00 | 3.41 | 55.04 ± 3.26 |
| 8 | 48.00 | 4.00 | 55.47 ± 2.30 |
| 9 | 82.00 | 0.59 | 55.38 ± 1.20 |
| 10 | 0.00 | 2.00 | 55.12 ± 2.72 |
| 11 | 48.00 | 2.00 | 65.89 ± 3.29 |
| 12 | 48.00 | 2.00 | 65.42 ± 3.85 |
| 13 | 82.00 | 3.41 | 55.98 ± 1.01 |
| 14 | 82.00 | 0.59 | 54.27 ± 2.86 |
| 15 | 14.00 | 3.41 | 54.12 ± 2.80 |
| 16 | 48.00 | 2.00 | 65.54 ± 1.57 |
| 17 | 96.00 | 2.00 | 58.23 ± 1.81 |
| 18 | 48.00 | 2.00 | 65.02 ± 1.65 |
| 19 | 48.00 | 2.00 | 65.99 ± 2.35 |
| 20 | 96.00 | 2.00 | 58.74 ± 4.67 |
| 21 | 14.00 | 0.59 | 55.74 ± 1.88 |
| Source | Sum of Squares | DF | Mean Squares | F-Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 370.24 | 5 | 74.05 | 66.39 | <0.0001 |
| Residual | 16.73 | 15 | 1.12 | ||
| Lack of fit | 13.98 | 3 | 4.66 | 20.37 | <0.0001 |
| Pure error | 2.75 | 12 | 0.23 | ||
| Cor total | 386.97 | 20 |
| Factor | Coefficient Estimate | Standard Error | %95CI Low | %95CI High | F-Value | p-Value (Prob > F) |
|---|---|---|---|---|---|---|
| Intercept | 65.57 | 0.47 | 64.57 | 66.58 | ||
| A: Time | 0.57 | 0.26 | 0.010 | 1.14 | 4.71 | 0.0465 |
| B: Concentration | −0.26 | 0.26 | −0.82 | 0.30 | 0.98 | 0.3381 |
| AB | 0.46 | 0.37 | −0.33 | 1.26 | 1.54 | 0.2333 |
| A2 | −4.66 | 0.33 | −5.36 | −3.96 | 200.91 | <0.0001 |
| B2 | −5.07 | 0.33 | −5.77 | −4.37 | 238.18 | <0.0001 |
| Compound | Regression Equation | R2 | Linear Rage (mg/mL) |
|---|---|---|---|
| Isoquercitrin | Y = 107X + 20,031 | 0.9998 | 0.00625–0.1 |
| Rutin | Y = 3 × 106X + 63,753 | 0.9995 | 0.1–2 |
| Narcissoside | Y = 7 × 106X − 11,287 | 0.9994 | 0.025–0.5 |
| Kaempferol-3-rutinoside | Y = 107X + 12,755 | 0.9994 | 0.0125–0.2 |
| Quercetin | Y = 5 × 106X + 44,515 | 0.9961 | 0.00625–0.2 |
| Sakuranetin | Y = 106X − 813.67 | 0.9988 | 0.0125–0.2 |
| Compound | Original Content (mg) | Addition (mg) | Total Content (mg) | Return (%) | Mean Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|
| Isoquercitrin | 0.152 | 0.122 | 0.270 | 98.54 | 97.24 | 1.41 |
| 0.152 | 0.152 | 0.296 | 97.37 | |||
| 0.152 | 0.182 | 0.320 | 95.81 | |||
| Rutin | 1.142 | 0.914 | 1.990 | 96.80 | 6.90 | 1.84 |
| 1.142 | 1.142 | 2.174 | 95.18 | |||
| 1.142 | 1.370 | 2.480 | 98.73 | |||
| Narcissoside | 0.308 | 0.246 | 0.544 | 98.19 | 97.46 | 1.15 |
| 0.308 | 0.308 | 0.596 | 98.03 | |||
| 0.308 | 0.370 | 0.652 | 96.17 | |||
| Kaempferol-3-rutinoside | 0.104 | 0.084 | 0.180 | 95.74 | 97.04 | 1.30 |
| 0.104 | 0.104 | 0.202 | 97.12 | |||
| 0.104 | 0.124 | 0.224 | 98.25 | |||
| Quercetin | 0.096 | 0.076 | 0.170 | 98.84 | 97.66 | 1.35 |
| 0.096 | 0.096 | 0.188 | 97.92 | |||
| 0.096 | 0.116 | 0.204 | 96.23 | |||
| Sakuranetin | 0.830 | 0.664 | 1.448 | 96.92 | 96.76 | 1.67 |
| 0.830 | 0.830 | 1.632 | 98.31 | |||
| 0.830 | 0.996 | 1.736 | 95.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Chen, H.; Xu, B.; Tan, Y.; Ling, Q.; Shi, L. Citric Acid Changes the Fingerprint of Flavonoids and Promotes Their Accumulation in Phellinus igniarius (L.) Quél. Life 2023, 13, 68. https://doi.org/10.3390/life13010068
Dong H, Chen H, Xu B, Tan Y, Ling Q, Shi L. Citric Acid Changes the Fingerprint of Flavonoids and Promotes Their Accumulation in Phellinus igniarius (L.) Quél. Life. 2023; 13(1):68. https://doi.org/10.3390/life13010068
Chicago/Turabian StyleDong, Haoran, Hui Chen, Bing Xu, Yingru Tan, Qun Ling, and Liang Shi. 2023. "Citric Acid Changes the Fingerprint of Flavonoids and Promotes Their Accumulation in Phellinus igniarius (L.) Quél" Life 13, no. 1: 68. https://doi.org/10.3390/life13010068