Immune Responses of the Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae) Reared on Catering Waste
Abstract
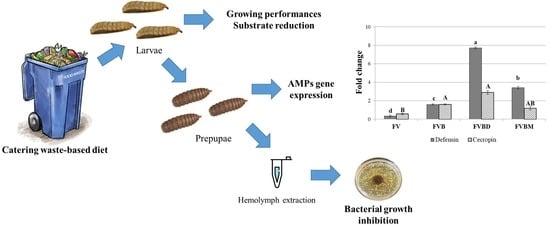
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Gene Expression Analysis
Quantitative Real-Time PCR
2.3. Hemolymph Inhibitory Activity
2.4. Statistical Analysis
3. Results
3.1. BSF Growing Performances and Substrate Reduction
3.2. Gene Expression Analysis
3.3. Hemolyomph Inhibitory Activity
3.3.1. Escherichia coli DH5α
3.3.2. Micrococcus yunnanensis HI55
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Environment Programme. Food Waste Index Report 2021; UNEP: Nairobi, Kenya, 2021; Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 20 November 2022).
- Wu, K.C.; Yau, Y.H.; Sze, E.T.P. Application of anaerobic bacterial ammonification pretreatment to microalgal food waste leachate cultivation and biofuel production. Mar. Pollut. Bull. 2020, 153, 111007. [Google Scholar] [CrossRef] [PubMed]
- Mbow, C.; Rosenzweig, C.; Barioni, L.G.; Benton, T.G.; Herrero, M.; Krishnapillai, M.; Liwenga, E.; Pradhan, P.; Rivera-Ferre, M.G.; Sapkota, T.; et al. Food security. In Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019; p. 200. [Google Scholar]
- Pahmeyer, M.J.; Siddiqui, S.A.; Pleissner, D.; Gołaszewski, J.; Heinz, V.; Smetana, S. An automated, modular system for organic waste utilization using Hermetia illucens larvae: Design, sustainability, and economics. J. Clean. Prod. 2022, 379, 134727. [Google Scholar] [CrossRef]
- Thi, N.B.D.; Kumar, G.; Lin, C.Y. An overview of food waste management in developing countries: Current status and future perspective. J. Environ. Manag. 2015, 157, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security (FAO Forestry Paper No. 171); Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Available online: https://edepot.wur.nl/258042 (accessed on 25 November 2022).
- Cheng, J.Y.; Chiu, S.L.; Lo, I.M. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef]
- van Huis, A.; Oonincx, D.G. The environmental sustainability of insects as food and feed. A review. Agron. Sustain. Dev. 2017, 37, 43. [Google Scholar] [CrossRef] [Green Version]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food waste valorisation and circular economy concepts in insect production and processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef]
- Ravi, H.K.; Degrou, A.; Costil, J.; Trespeuch, C.; Chemat, F.; Vian, M.A. Larvae mediated valorization of industrial, agriculture and food wastes: Biorefinery concept through bioconversion, processes, procedures, and products. Processes 2020, 8, 857. [Google Scholar] [CrossRef]
- International Platform of Insects for Food and Feed (IPIFF). An Overview of the European Market of Insects as Feed. 2022. Available online: https://ipiff.org/wp-content/uploads/2021/04/apr-27-2021-ipiff_the-european-market-of-insects-as-feed.pdf (accessed on 25 November 2022).
- Fowles, T.M.; Nansen, C. Insect-Based bioconversion: Value from food waste. In Food Waste Management; Närvänen, E., Mesiranta, N., Mattila, M., Heikkinen, A., Eds.; Palgrave Macmillan: London, UK, 2020; pp. 321–346. [Google Scholar]
- Wang, Y.S.; Shelomi, M. Review of black soldier fly (Hermetia illucens) as animal feed and human food. Foods 2017, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Jiang, C.; Zhang, Z.; Lu, W.; Wang, H. Material flow analysis and life cycle assessment of food waste bioconversion by black soldier fly larvae (Hermetia illucens L.). Sci. Total Environ. 2021, 750, 141656. [Google Scholar] [CrossRef]
- Kim, C.H.; Ryu, J.; Lee, J.; Ko, K.; Lee, J.Y.; Park, K.Y.; Chung, H. Use of black soldier fly larvae for food waste treatment and energy production in Asian countries: A review. Processes 2021, 9, 161. [Google Scholar] [CrossRef]
- Lu, J.; Guo, Y.; Muhmood, A.; Zeng, B.; Qiu, Y.; Wang, P.; Ren, L. Probing the antioxidant activity of functional proteins and bioactive peptides in Hermetia illucens larvae fed with food wastes. Sci. Rep. 2022, 12, 2799. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Ability of black soldier fly (Diptera: Stratiomyidae) larvae to recycle food waste. Environ. Entomol. 2015, 44, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia iIllucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Renna, M.; Schiavone, A.; Gai, F.; Dabbou, S.; Lussiana, C.; Malfatto, V.; Prearo, M.; Capucchio, M.T.; Biasato, I.; Biasibetti, E.; et al. Evaluation of the suitability of a partially defatted black soldier fly (Hermetia illucens L.) larvae meal as ingredient for rainbow trout (Oncorhynchus mykiss Walbaum) diets. J. Anim. Sci. Biotechnol. 2017, 8, 57. [Google Scholar] [PubMed]
- Xu, X.X.; Ji, H.; Yu, H.B.; Zhou, J.S. Influence of dietary black soldier fly (Hermetia illucens Linnaeus) pulp on growth performance, antioxidant capacity and intestinal health of juvenile mirror carp (Cyprinus carpio Var. Specularis). Aquac. Nutr. 2020, 26, 432–443. [Google Scholar] [CrossRef]
- Caimi, C.; Biasato, I.; Chemello, G.; Oddon, S.B.; Lussiana, C.; Malfatto, V.M.; Capucchio, M.T.; Colombino, E.; Schiavone, A.; Gai, F.; et al. Dietary inclusion of a partially defatted black soldier fly (Hermetia iIllucens) larva meal in low fishmeal-based diets for rainbow trout (Oncorhynchus mykiss). J. Anim. Sci. Biotechnol. 2021, 12, 50. [Google Scholar] [CrossRef]
- Takakuwa, F.; Tanabe, R.; Nomura, S.; Inui, T.; Yamada, S.; Biswas, A.; Tanaka, H. Availability of black soldier fly meal as an alternative protein source to fish meal in red sea bream (Pagrus major, Temminck & Schlegel) fingerling diets. Aquac. Res. 2021, 53, 36–49. [Google Scholar]
- Terova, G.; Rimoldi, S.; Ascione, C.; Gini, E.; Ceccotti, C.; Gasco, L. Rainbow trout (Oncorhynchus mykiss) gut microbiota is modulated by insect meal from Hermetia illucens prepupae in the diet. Rev. Fish Biol. Fish. 2019, 29, 465–486. [Google Scholar] [CrossRef]
- Lee, K.P.; Cory, J.S.; Wilson, K.; Raubenheimer, D.; Simpson, S.J. Flexible diet choice offsets protein costs of pathogen resistance in a caterpillar. Proc. Biol. Sci. 2006, 273, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Vogel, H.; Müller, A.; Heckel, D.G.; Gutzeit, H.; Vilcinskas, A. Nutritional immunology: Diversification and diet-dependent expression of antimicrobial peptides in the black soldier fly Hermetia illucens. Dev. Comp. Immunol. 2018, 78, 141–148. [Google Scholar] [CrossRef]
- Cammack, J.; Tomberlin, J. The impact of diet protein and carbohydrate on select life-history traits of the black soldier fly Hermetia illucens (L.) (Diptera: Stratiomyidae). Insects 2017, 8, 56. [Google Scholar] [CrossRef]
- Siva-Jothy, M.T.; Thompson, J.J. Short term nutrient deprivation affects immune function. Physiol. Entomol. 2002, 27, 206–212. [Google Scholar] [CrossRef]
- Catalán, T.P.; Barceló, M.; Niemeyer, H.M.; Kalergis, A.M.; Bozinovic, F. Pathogen-and diet-dependent foraging, nutritional and immune ecology in mealworms. Evol. Ecol. Res. 2011, 13, 711–723. [Google Scholar]
- De Smet, J.; Wynants, E.; Cos, P.; Van Campenhout, L. Microbial community dynamics during rearing of black soldier fly larvae (Hermetia illucens) and impact on exploitation potential. Appl. Environ. Microbiol. 2018, 84, e02722-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, D.; Bonelli, M.; De Filippis, F.; Di Lelio, I.; Tettamanti, G.; Casartelli, M.; Ercolini, D.; Caccia, S. The intestinal microbiota of Hermetia illucens larvae is affected by diet and shows a diverse composition in the different midgut regions. Appl. Environ. Microbiol. 2019, 85, e01864-18. [Google Scholar] [CrossRef] [Green Version]
- Candian, V.; Savio, C.; Meneguz, M.; Gasco, L.; Tedeschi, R. Effect of the rearing diet on gene expression of antimicrobial peptides in Hermetia illucens (Diptera: Stratiomyidae). Insect Sci. 2023; Accepted Author Manuscript. [Google Scholar] [CrossRef]
- Vyas, D.K.; Murphy, S.M. Diet-mediated immune response to parasitoid attacks on a caterpillar with a broad diet breadth. Ecol. Entomol. 2022, 7, 636–644. [Google Scholar] [CrossRef]
- Tomberlin, J.K.; Sheppard, D.C.; Joyce, J.A. Selected life-history traits of black soldier flies (Diptera: Stratiomyidae) reared on three artificial diets. Ann. Entomol. Soc. Am. 2002, 95, 379–386. [Google Scholar] [CrossRef]
- Shin, H.S.; Park, S.I. Novel attacin from Hermetia illucens: cDNA cloning, characterization, and antibacterial properties. Prep. Biochem. Biotechnol. 2019, 49, 279–285. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tabunoki, H.; Dittmer, N.T.; Gorman, M.J.; Kanost, M.R. Development of a new method for collecting hemolymph and measuring phenoloxidase activity in Tribolium castaneum. BMC Res. Notes 2019, 12, 7. [Google Scholar] [CrossRef]
- Callegari, M.; Jucker, C.; Fusi, M.; Leonardi, M.G.; Daffonchio, D.; Borin, S.; Savoldelli, S.; Crotti, E. Hydrolytic profile of the culturable gut bacterial community associated with Hermetia illucens. Front. Microbiol. 2020, 11, 1965. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: “Global Food Losses and Food Waste-Extent, Causes and Prevention”—FAO 2011; SIK Report No. 857; The Swedish Institute for Food and Biotechnology: Goteborg, Sweden, 2013; p. 29. Available online: https://www.diva-portal.org/smash/get/diva2:944159/fulltext01.pdf (accessed on 25 November 2022).
- Bava, L.; Jucker, C.; Gislon, G.; Lupi, D.; Savoldelli, S.; Zucali, M.; Colombini, S. Rearing of Hermetia illucens on different organic by-products: Influence on growth, waste reduction, and environmental impact. Animals 2019, 9, 289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.T.X.; Tomberlin, J.K.; Vanlaerhoven, S. Influence of resources on Hermetia illucens (Diptera: Stratiomyidae) larval development. J. Med. Entomol. 2013, 50, 898–906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef] [Green Version]
- Lalander, C.; Diener, S.; Zurbrügg, C.; Vinnerås, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Barragán-Fonseca, K.; Pineda-Mejia, J.; Dicke, M.; Van Loon, J.J. Performance of the black soldier fly (Diptera: Stratiomyidae) on vegetable residue-based diets formulated based on protein and carbohydrate contents. J. Econ. Entomol 2018, 111, 2676–2683. [Google Scholar] [CrossRef]
- Barragan-Fonseca, K.B.; Gort, G.; Dicke, M.; Van Loon, J.J.A. Nutritional plasticity of the black soldier fly (Hermetia illucens) in response to artificial diets varying in protein and carbohydrate concentrations. J. Insects Food Feed 2021, 7, 51–61. [Google Scholar] [CrossRef]
- Kawasaki, K.; Ohkawa, M.; Zhao, J.; Yano, K. Effect of dietary meat content on weight gain, mortality, and pre-pupal rate in black soldier fly (Hermetia illucens) larvae. Insects 2022, 13, 229. [Google Scholar] [CrossRef]
- Jucker, C.; Erba, D.; Leonardi, M.G.; Lupi, D.; Savoldelli, S. Assessment of vegetable and fruit substrates as potential rearing media for Hermetia illucens (Diptera: Stratiomyidae) larvae. Environ. Entomol 2017, 46, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Meneguz, M.; Schiavone, A.; Gai, F.; Dama, A.; Lussiana, C.; Renna, M.; Gasco, L. Effect of rearing substrate on growth performance, waste reduction efficiency and chemical composition of black soldier fly (Hermetia illucens) larvae. J. Sci. Food Agric. 2018, 98, 5776–5784. [Google Scholar] [CrossRef]
- International Platform of Insects for Food and Feed (IPIFF). Building Bridges between the Insect Production Chain, Research and Policymakers. 2022. Available online: https://ipiff.org/wp-content/uploads/2019/12/ipiff-researchpriorities-horizoneurope.pdf (accessed on 31 December 2022).
- Jagtap, S.; Garcia-Garcia, G.; Duong, L.; Swainson, M.; Martindale, W. Codesign of food system and circular economy approaches for the development of livestock feeds from insect larvae. Foods 2021, 10, 1701. [Google Scholar] [CrossRef] [PubMed]
- Krams, I.; Kecko, S.; Kangassalo, K.; Moore, F.R.; Jankevics, E.; Inashkina, I.; Krama, T.; Lietuvietis, V.; Meija, L.; Rantala, M.J. Effects of food quality on trade-offs among growth, immunity and survival in the greater wax moth Galleria mellonella. Insect Sci. 2015, 22, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Smits, S.A.; Leach, J.; Sonnenburg, E.D.; Gonzalez, C.G.; Lichtman, J.S.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Elias, J.E.; et al. Seasonal cycling in the gut microbiome of the Hadza hunter-gatherers of Tanzania. Science 2017, 357, 802–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, E.; Sasidharan, A.; Ode, P.J.; Venkatesan, R. Oviposition preference and performance of a specialist herbivore is modulated by natural enemies, larval odors, and immune status. J. Chem. Ecol. 2022, 48, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.Y.; Chowdhury, M.; Huang, Y.D.; Yu, X.Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef] [Green Version]
- Biasato, I.; Chemello, G.; Oddon, S.B.; Ferrocino, I.; Corvaglia, M.R.; Caimi, C.; Resconi, A.; Paul, A.; van Spankeren, M.; Capucchio, M.T.; et al. Hermetia illucens meal inclusion in low-fishmeal diets for rainbow trout (Oncorhynchus mykiss): Effects on the growth performance, nutrient digestibility coefficients, selected gut health traits, and health status indices. Anim. Feed Sci. Technol. 2022, 290, 115341. [Google Scholar] [CrossRef]
- López-Uribe, M.M.; Appler, R.H.; Youngsteadt, E.; Dunn, R.R.; Frank, S.D.; Tarpy, D.R. Higher immunocompetence is associated with higher genetic diversity in feral honey bee colonies (Apis mellifera). Conserv. Genet. 2017, 18, 659–666. [Google Scholar] [CrossRef]
- Choi, W.H.; Choi, H.J.; Goo, T.W.; Quan, F.S. Novel antibacterial peptides induced by probiotics in Hermetia illucens (Diptera: Stratiomyidae) larvae. Entomol. Res. 2018, 48, 237–247. [Google Scholar] [CrossRef]
- Bruno, D.; Montali, A.; Mastore, M.; Brivio, M.F.; Mohamed, A.; Tian, L.; Grimaldi, A.; Casartelli, M.; Tettamanti, G. Insights into the immune response of the black soldier fly larvae to bacteria. Front. Immunol. 2021, 12, 745160. [Google Scholar] [CrossRef]
- Karataş, A. Dairy products added to rearing media negatively effect Drosophila melanogaster (Diptera: Drosophilidae) egg production and larval development. J. Insect Sci. 2018, 18, 7. [Google Scholar]
- Pastor, B.; Kova, H.; Kozanek, M.; Martinez-Sanchez, A.; Taka, P.; Rojo, S. Effect of the size of the pupae, adult diet, oviposition substrate and adult population density on egg production in Musca domestica (Diptera: Muscidae). Eur. J. Entomol. 2011, 108, 587–596. [Google Scholar] [CrossRef] [Green Version]
- Blow, F.; Douglas, A.E. The hemolymph microbiome of insects. J. Insect Physiol. 2019, 115, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Lee, B.L.; Soderhall, K. The proPO-system: Pros and cons for its role in invertebrate immunity. Trends Immunol. 2008, 29, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef]
- Shokal, U.; Eleftherianos, I. Evolution and function of thioester-containing proteins and the complement system in the innate immune response. Front. Immunol. 2017, 8, 759. [Google Scholar] [CrossRef] [Green Version]
- Park, S.I.; Kim, J.W.; Yoe, S.M. Purification and characterization of a novel antibacterial peptide from black soldier fly (Hermetia illucens) larvae. Dev. Comp. Immunol. 2015, 52, 98–106. [Google Scholar] [CrossRef]
- Lee, D.H.; Chu, K.B.; Kang, H.J.; Lee, S.H.; Quan, F.S. Peptides in the hemolymph of Hermetia illucens larvae completely inhibit the growth of Klebsiella pneumonia in vitro and in vivo. J. Asia Pac. Entomol. 2020, 23, 36–43. [Google Scholar] [CrossRef]
- Lee, K.S.; Yun, E.Y.; Goo, T.W. Antimicrobial activity of an extract of Hermetia illucens larvae immunized with Lactobacillus casei against Salmonella species. Insects 2020, 11, 704. [Google Scholar] [CrossRef]
- Lee, K.S.; Yun, E.Y.; Goo, T.W. Evaluation of the antimicrobial activity of an extract of Lactobacillus casei-infected Hermetia illucens larvae produced using an automatic injection system. Animals 2020, 10, 2121. [Google Scholar] [CrossRef]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, A.; Habineza, P.; Ji, T.; Hou, Y.; Shi, Z. Intestinal microbiota confer protection by priming the immune system of red palm weevil Rhynchophorus ferrugineus Olivier (Coleoptera: Dryophthoridae). Front. Physiol. 2019, 10, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Ceron, L.; Santillan, F.; Rodriguez, M.H.; Mendez, D.; Hernandez-Avila, J.E. Bacteria in midguts of field-collected Anopheles albimanus block Plasmodium vivax sporogonic development. J. Med. Entomol. 2003, 40, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Cirimotich, C.M.; Dong, Y.; Clayton, A.M.; Sandiford, S.L.; Souza-Neto, J.A.; Mulenga, M.; Dimopoulos, G. Natural microbe-mediated refractoriness to Plasmodium infection in Anopheles gambiae. Science 2011, 332, 855–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, I.; Damiani, C.; Capone, A.; DeFreece, C.; Rossi, P.; Favia, G. Mosquito/microbiota interactions: From complex relationships to biotechnological perspectives. Curr. Opin. Microbiol. 2012, 15, 278–284. [Google Scholar] [CrossRef] [PubMed]





| Parameter | NatOvo | FV | FVB | FVBD | FVBM |
|---|---|---|---|---|---|
| Final weight (mg) | 181.10 ± 5.71 a | 115.43 ± 3.10 c | 148.18 ± 1.95 b | 149.33 ± 2.33 b | 176.80 ± 2.00 a |
| ADG (mg day−1) | 23.01 ± 0.81 a | 13.49 ± 0.44 c | 18.17 ± 0.28 b | 18.33 ± 0.33 b | 22.26 ± 0.29 a |
| Survival rate (%) | 75.54 ± 2.67 d | 97.19 ± 0.31 a | 82.44 ± 0.29 c | 88.11 ± 1.07 b | 76.80 ± 1.25 d |
| Final larval biomass (mg) | 274.50 ± 1.18 a | 224.40 ± 6.50 c | 244.30 ± 2.44 b | 263.07 ± 2.00 a | 271.47 ± 1.75 a |
| Substrate reduction (%) | 86.33 ± 0.38 a | 64.33 ± 2.60 d | 76.90 ± 0.38 b | 73.87 ± 0.26 c | 76.10 ± 0.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candian, V.; Meneguz, M.; Tedeschi, R. Immune Responses of the Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae) Reared on Catering Waste. Life 2023, 13, 213. https://doi.org/10.3390/life13010213
Candian V, Meneguz M, Tedeschi R. Immune Responses of the Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae) Reared on Catering Waste. Life. 2023; 13(1):213. https://doi.org/10.3390/life13010213
Chicago/Turabian StyleCandian, Valentina, Marco Meneguz, and Rosemarie Tedeschi. 2023. "Immune Responses of the Black Soldier Fly Hermetia illucens (L.) (Diptera: Stratiomyidae) Reared on Catering Waste" Life 13, no. 1: 213. https://doi.org/10.3390/life13010213