Analysis of Prevalence and Predictive Factors of Long-Lasting Olfactory and Gustatory Dysfunction in COVID-19 Patients
Abstract
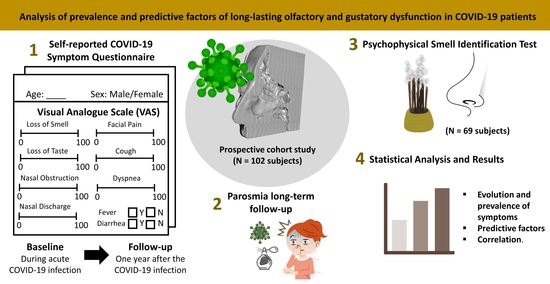
:1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Participants
2.2. Study Variables
2.3. Data Analysis and Statistical Methods
3. Results
3.1. Evolution of COVID-19 Symptoms
3.2. Prevalence, Severity, and Time of Recovery of Chemosensory Dysfunction
3.3. Prevalence of Parosmia after COVID-19 Infection
3.4. Psychophysical Evaluation of Olfactory Function
4. Discussion
4.1. Prevalence of Chemosensory Dysfunction in COVID-19 Subjects
4.2. Evolution, Severity, and Time of Recovery of Chemosensory Dysfunction
4.3. Prevalence of Parosmia 12 Months after COVID-19 Infection
4.4. Psychophysical (UPSIT) Evaluation of Olfactory Function 12 Months after COVID-19 Infection
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, H.; Asseo, K.; Karni, N.; Benjamini, Y.; Nir-Paz, R.; Muszkat, M.; Israel, S.; Niv, M.Y. Onset, Duration and Unresolved Symptoms, Including Smell and Taste Changes, in Mild COVID-19 Infection: A Cohort Study in Israeli Patients. Clin. Microbiol. Infect. 2021, 27, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Moein, S.T.; Hashemian, S.M.; Mansourafshar, B.; Khorram-Tousi, A.; Tabarsi, P.; Doty, R.L. Smell Dysfunction: A Biomarker for COVID-19. Int. Forum Allergy Rhinol. 2020, 10, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.Y.; Wong, A.; Zhu, D.; Fastenberg, J.H.; Tham, T. The Prevalence of Olfactory and Gustatory Dysfunction in COVID-19 Patients: A Systematic Review and Meta-Analysis. Otolaryngol. Head Neck Surg. 2020, 163, 3–11. [Google Scholar] [CrossRef]
- Da Costa, K.V.; Carnaúba, A.T.L.; Rocha, K.W.; de Andrade, K.C.L.; Ferreira, S.M.; Menezes, P.D.L. Olfactory and Taste Disorders in COVID-19: A Systematic Review. Braz. J. Otorhinolaryngol. 2020, 86, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Vaira, L.A.; Salzano, G.; Deiana, G.; De Riu, G. Anosmia and Ageusia: Common Findings in COVID-19 Patients. Laryngoscope 2020, 130, 1787. [Google Scholar] [CrossRef]
- Callejon-Leblic, M.A.; Moreno-Luna, R.; Del Cuvillo, A.; Reyes-Tejero, I.M.; Garcia-Villaran, M.A.; Santos-Peña, M.; Maza-Solano, J.M.; Martín-Jimenez, D.I.; Palacios-Garcia, J.M.; Fernandez-Velez, C.; et al. Loss of Smell and Taste Can Accurately Predict COVID-19 Infection: A Machine-Learning Approach. J. Clin. Med. 2021, 10, 570. [Google Scholar] [CrossRef]
- Butowt, R.; von Bartheld, C.S. Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. Neuroscientist 2021, 27, 582–603. [Google Scholar] [CrossRef]
- Xydakis, M.S.; Albers, M.W.; Holbrook, E.H.; Lyon, D.M.; Shih, R.Y.; Frasnelli, J.A.; Pagenstecher, A.; Kupke, A.; Enquist, L.W.; Perlman, S. Post-Viral Effects of COVID-19 in the Olfactory System and Their Implications. Lancet Neurol. 2021, 20, 753–761. [Google Scholar] [CrossRef]
- Hopkins, C.; Surda, P.; Vaira, L.A.; Lechien, J.R.; Safarian, M.; Saussez, S.; Kumar, N. Six Month Follow-up of Self-Reported Loss of Smell during the COVID-19 Pandemic. Rhinology 2021, 59, 26–31. [Google Scholar] [CrossRef]
- Boscolo-Rizzo, P.; Menegaldo, A.; Fabbris, C.; Spinato, G.; Borsetto, D.; Vaira, L.A.; Calvanese, L.; Pettorelli, A.; Sonego, M.; Frezza, D.; et al. Six-Month Psychophysical Evaluation of Olfactory Dysfunction in Patients with COVID-19. Chem. Senses 2021, 46, bjab006. [Google Scholar] [CrossRef]
- Walker, A.; Kelly, C.; Pottinger, G.; Hopkins, C. Parosmia—A Common Consequence of COVID-19. BMJ 2022, 377, e069860. [Google Scholar] [CrossRef] [PubMed]
- Haehner, A.; Marquardt, B.; Kardashi, R.; de With, K.; Rößler, S.; Landis, B.N.; Welge-Luessen, A.; Hummel, T. SARS-CoV-2 Leads to Significantly More Severe Olfactory Loss than Other Seasonal Cold Viruses. Life 2022, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Nordin, S.; Brämerson, A.; Millqvist, E.; Bende, M. Prevalence of Parosmia: The Skövde Population-Based Studies. Rhinology 2007, 45, 50–53. [Google Scholar]
- Patel, Z.M.; Holbrook, E.H.; Turner, J.H.; Adappa, N.D.; Albers, M.W.; Altundag, A.; Appenzeller, S.; Costanzo, R.M.; Croy, I.; Davis, G.E.; et al. International Consensus Statement on Allergy and Rhinology: Olfaction. Int. Forum Allergy Rhinol. 2022, 12, 327–680. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.A.; Alaqeedy, A.A.; Al-Ani, R.M. Parosmia Due to COVID-19 Disease: A 268 Case Series. Indian J. Otolaryngol. Head Neck Surg. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Polesel, J.; Spinato, G.; Menegaldo, A.; Fabbris, C.; Calvanese, L.; Borsetto, D.; Hopkins, C. Predominance of an Altered Sense of Smell or Taste among Long-Lasting Symptoms in Patients with Mildly Symptomatic COVID-19. Rhinology 2020, 58, 524–525. [Google Scholar] [CrossRef] [PubMed]
- Nehme, M.; Braillard, O.; Chappuis, F.; Courvoisier, D.S.; Guessous, I.; CoviCare Study Team. Prevalence of Symptoms More Than Seven Months After Diagnosis of Symptomatic COVID-19 in an Outpatient Setting. Ann. Intern. Med. 2021, 174, 1252–1260. [Google Scholar] [CrossRef]
- Lim, M.; Lew-Gor, S.; Darby, Y.; Brookes, N.; Scadding, G.; Lund, V.J. The Relationship between Subjective Assessment Instruments in Chronic Rhinosinusitis. Rhinology 2007, 45, 144–147. [Google Scholar]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58 (Suppl. S29), 1–464. [Google Scholar] [CrossRef]
- Doty, R.L.; Frye, R.E.; Agrawal, U. Internal Consistency Reliability of the Fractionated and Whole University of Pennsylvania Smell Identification Test. Percept. Psychophys. 1989, 45, 381–384. [Google Scholar] [CrossRef]
- Doty, R.L.; Agrawal, U. The Shelf Life of the University of Pennsylvania Smell Identification Test (UPSIT). Laryngoscope 1989, 99, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Chiesa-Estomba, C.M.; Lechien, J.R.; Radulesco, T.; Michel, J.; Sowerby, L.J.; Hopkins, C.; Saussez, S. Patterns of Smell Recovery in 751 Patients Affected by the COVID-19 Outbreak. Eur. J. Neurol. 2020, 27, 2318–2321. [Google Scholar] [CrossRef] [PubMed]
- Saussez, S.; Sharma, S.; Thiriard, A.; Olislagers, V.; Vu Duc, I.; Le Bon, S.; Khalife, M.; Hans, S.; De Riu, G.; Hopkins, C.; et al. Predictive Factors of Smell Recovery in a Clinical Series of 288 Coronavirus Disease 2019 Patients with Olfactory Dysfunction. Eur. J. Neurol. 2021, 28, 3702–3711. [Google Scholar] [CrossRef] [PubMed]
- Chary, E.; Carsuzaa, F.; Trijolet, J.-P.; Capitaine, A.-L.; Roncato-Saberan, M.; Fouet, K.; Cazenave-Roblot, F.; Catroux, M.; Allix-Beguec, C.; Dufour, X. Prevalence and Recovery from Olfactory and Gustatory Dysfunctions in Covid-19 Infection: A Prospective Multicenter Study. Am. J. Rhinol. Allergy 2020, 34, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Speth, M.M.; Singer-Cornelius, T.; Oberle, M.; Gengler, I.; Brockmeier, S.J.; Sedaghat, A.R. Time Scale for Resolution of Olfactory Dysfunction in COVID-19. Rhinnology 2020, 58, 404–405. [Google Scholar] [CrossRef] [PubMed]
- Riestra-Ayora, J.; Yanes-Diaz, J.; Esteban-Sanchez, J.; Vaduva, C.; Molina-Quiros, C.; Larran-Jimenez, A.; Martin-Sanz, E. Long-Term Follow-up of Olfactory and Gustatory Dysfunction in COVID-19: 6 Months Case–Control Study of Health Workers. Eur. Arch. Otorhinolaryngol. 2021, 278, 4831–4837. [Google Scholar] [CrossRef]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; DeConde, A.S. Association of Chemosensory Dysfunction and COVID-19 in Patients Presenting with Influenza-like Symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef]
- Salcan, İ.; Karakeçili, F.; Salcan, S.; Ünver, E.; Akyüz, S.; Seçkin, E.; Cingi, C. Is Taste and Smell Impairment Irreversible in COVID-19 Patients? Eur. Arch. Otorhinolaryngol. 2021, 278, 411–415. [Google Scholar] [CrossRef]
- Vaira, L.A.; Hopkins, C.; Salzano, G.; Petrocelli, M.; Melis, A.; Cucurullo, M.; Ferrari, M.; Gagliardini, L.; Pipolo, C.; Deiana, G.; et al. Olfactory and Gustatory Function Impairment in COVID -19 Patients: Italian Objective Multicenter-study. Head Neck 2020, 42, 1560–1569. [Google Scholar] [CrossRef]
- Izquierdo-Domínguez, A.; Rojas-Lechuga, M.J.; Chiesa-Estomba, C.; Calvo-Henríquez, C.; Ninchritz-Becerra, E.; Soriano-Reixach, M.; Poletti-Serafini, D.; Villarreal, I.M.; Maza-Solano, J.M.; Moreno-Luna, R.; et al. Smell and Taste Dysfunction in COVID-19 Is Associated with Younger Age in Ambulatory Settings: A Multicenter Cross-Sectional Study. J. Investig. Allergol. Clin. Immunol. 2020, 30, 346–357. [Google Scholar] [CrossRef]
- Brandão Neto, D.; Fornazieri, M.A.; Dib, C.; Di Francesco, R.C.; Doty, R.L.; Voegels, R.L.; Pinna, F.D.R. Chemosensory Dysfunction in COVID-19: Prevalences, Recovery Rates, and Clinical Associations on a Large Brazilian Sample. Otolaryngol. Head Neck Surg. 2021, 164, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Bussière, N.; Mei, J.; Lévesque-Boissonneault, C.; Blais, M.; Carazo, S.; Gros-Louis, F.; De Serres, G.; Dupré, N.; Frasnelli, J. Chemosensory Dysfunctions Induced by COVID-19 Can Persist up to 7 Months: A Study of over 700 Healthcare Workers. Chem. Senses 2021, 46, bjab038. [Google Scholar] [CrossRef] [PubMed]
- Boscolo-Rizzo, P.; Guida, F.; Polesel, J.; Marcuzzo, A.V.; Antonucci, P.; Capriotti, V.; Sacchet, E.; Cragnolini, F.; D’Alessandro, A.; Zanelli, E.; et al. Self-Reported Smell and Taste Recovery in Coronavirus Disease 2019 Patients: A One-Year Prospective Study. Eur. Arch. Otorhinolaryngol. 2022, 279, 515–520. [Google Scholar] [CrossRef]
- Schwab, J.; Jensen, C.D.; Fjaeldstad, A.W. Sustained Chemosensory Dysfunction during the COVID-19 Pandemic. ORL 2021, 83, 209–218. [Google Scholar] [CrossRef]
- Printza, A.; Katotomichelakis, M.; Valsamidis, K.; Metallidis, S.; Panagopoulos, P.; Panopoulou, M.; Petrakis, V.; Constantinidis, J. Smell and Taste Loss Recovery Time in COVID-19 Patients and Disease Severity. J. Clin. Med. 2021, 10, 966. [Google Scholar] [CrossRef]
- Lechien, J.R.; Chiesa-Estomba, C.M.; De Siati, D.R.; Horoi, M.; Le Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; El Afia, F.; Distinguin, L.; et al. Olfactory and Gustatory Dysfunctions as a Clinical Presentation of Mild-to-Moderate Forms of the Coronavirus Disease (COVID-19): A Multicenter European Study. Eur. Arch. Otorhinolaryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef]
- Bertlich, M.; Stihl, C.; Lüsebrink, E.; Hellmuth, J.C.; Scherer, C.; Freytag, S.; Spiegel, J.L.; Stoycheva, I.; Canis, M.; Weiss, B.G.; et al. The Course of Subjective and Objective Chemosensory Dysfunction in Hospitalized Patients with COVID-19: A 6-Month Follow-Up. Eur. Arch. Otorhinolaryngol. 2021, 278, 4855–4861. [Google Scholar] [CrossRef]
- Fortunato, F.; Martinelli, D.; Iannelli, G.; Milazzo, M.; Farina, U.; Di Matteo, G.; De Nittis, R.; Ascatigno, L.; Cassano, M.; Lopalco, P.L.; et al. Self-Reported Olfactory and Gustatory Dysfunctions in COVID-19 Patients: A 1-Year Follow-up Study in Foggia District, Italy. BMC Infect. Dis. 2022, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Ohla, K.; Veldhuizen, M.G.; Green, T.; Hannum, M.E.; Bakke, A.J.; Moein, S.T.; Tognetti, A.; Postma, E.M.; Pellegrino, R.; Hwang, D.L.D.; et al. A Follow-up on Quantitative and Qualitative Olfactory Dysfunction and Other Symptoms in Patients Recovering from COVID-19 Smell Loss. Rhinology 2022. [Google Scholar] [CrossRef]
- Petrocelli, M.; Cutrupi, S.; Salzano, G.; Maglitto, F.; Salzano, F.A.; Lechien, J.R.; Saussez, S.; Boscolo-Rizzo, P.; De Riu, G.; Vaira, L.A. Six-Month Smell and Taste Recovery Rates in Coronavirus Disease 2019 Patients: A Prospective Psychophysical Study. J. Laryngol. Otol. 2021, 135, 436–441. [Google Scholar] [CrossRef]
- Jafar, A.; Lasso, A.; Shorr, R.; Hutton, B.; Kilty, S. Olfactory Recovery Following Infection with COVID-19: A Systematic Review. PLoS ONE 2021, 16, e0259321. [Google Scholar] [CrossRef] [PubMed]
- Coelho, D.H.; Reiter, E.R.; Budd, S.G.; Shin, Y.; Kons, Z.A.; Costanzo, R.M. Predictors of Smell Recovery in a Nationwide Prospective Cohort of Patients with COVID-19. Am. J. Otolaryngol. 2022, 43, 103239. [Google Scholar] [CrossRef] [PubMed]
- Jalessi, M.; Bagheri, S.H.; Azad, Z.; Firouzabadi, F.D.; Amini, E.; Alizadeh, R.; Chaibakhsh, S.; Ghalehbaghi, B.; Hopkins, C.; Farhadi, M. The Outcome of Olfactory Impairment in Patients with Otherwise Paucisymptomatic Coronavirus Disease 2019 during the Pandemic. J. Laryngol. Otol. 2021, 135, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Babaei, A.; Iravani, K.; Malekpour, B.; Golkhar, B.; Soltaniesmaeili, A.; Hosseinialhashemi, M. Factors Associated with Anosmia Recovery Rate in COVID-19 Patients. Laryngoscope Investig. Otolaryngol. 2021, 6, 1248–1255. [Google Scholar] [CrossRef]
- Bianco, M.R.; Ralli, M.; Minni, A.; Greco, A.; de Vincentiis, M.; Allegra, E. Evaluation of Olfactory Dysfunction Persistence after COVID-19: A Prospective Study. Eur. Rev. Med. Pharm. Sci. 2022, 26, 1042–1048. [Google Scholar] [CrossRef]
- Tognetti, A.; Thunell, E.; Olsson, M.J.; Greilert, N.; Havervall, S.; Thålin, C.; Lundström, J.N. High Prevalence of Olfactory Disorders 18 Months after Contracting COVID-19. Otolaryngology 2022. [Google Scholar] [CrossRef]
- Lerner, D.K.; Garvey, K.L.; Arrighi-Allisan, A.E.; Filimonov, A.; Filip, P.; Shah, J.; Tweel, B.; Del Signore, A.; Schaberg, M.; Colley, P.; et al. Clinical Features of Parosmia Associated With COVID-19 Infection. Laryngoscope 2022, 132, 633–639. [Google Scholar] [CrossRef]
- Duyan, M.; Ozturan, I.U.; Altas, M. Delayed Parosmia Following SARS-CoV-2 Infection: A Rare Late Complication of COVID-19. SN Compr. Clin. Med. 2021, 3, 1200–1202. [Google Scholar] [CrossRef]
- Karamali, K.; Elliott, M.; Hopkins, C. COVID-19 Related Olfactory Dysfunction. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 19–25. [Google Scholar] [CrossRef]
- Tham, A.C.; Thein, T.-L.; Lee, C.S.; Tan, G.S.E.; Manauis, C.M.; Siow, J.K.; Leo, Y.S.; Lim, M.Y. Olfactory Taste Disorder as a Presenting Symptom of COVID-19: A Large Single-Center Singapore Study. Eur. Arch. Otorhinolaryngol. 2021, 278, 1853–1862. [Google Scholar] [CrossRef]
- Ferreli, F.; Gaino, F.; Russo, E.; Di Bari, M.; Rossi, V.; De Virgilio, A.; Di Stadio, A.; Spriano, G.; Mercante, G. Long-term Olfactory Dysfunction in COVID-19 Patients: 18-month Follow-up Study. Int. Forum Allergy Rhinol. 2022, 12, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Hummel, T.; Hugentobler, M.; Giger, R.; Lacroix, J.S. Ratings of Overall Olfactory Function. Chem. Senses 2003, 28, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Kakutani, Y.; Narumi, T.; Kobayakawa, T.; Kawai, T.; Kusakabe, Y.; Kunieda, S.; Wada, Y. Taste of Breath: The Temporal Order of Taste and Smell Synchronized with Breathing as a Determinant for Taste and Olfactory Integration. Sci. Rep. 2017, 7, 8922. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.; Han, P.; Burghardt, S.; Knaapila, A.; Schriever, V.; Hummel, T. Influence of Olfactory Dysfunction on the Perception of Food. Eur. Arch. Otorhinolaryngol. 2019, 276, 2811–2817. [Google Scholar] [CrossRef]
- González, C.; García-Huidobro, F.G.; Lagos, A.E.; Aliaga, R.; Fuentes-López, E.; Díaz, L.A.; García-Salum, T.; Salinas, E.; Toro, A.; Callejas, C.A.; et al. Prospective Assessment of Smell and Taste Impairment in a South-American Coronavirus Disease 2019 (COVID-19) Cohort: Association with the Need for Hospitalization and Reversibility of Dysfunction. Int. Forum Allergy Rhinol. 2021, 11, 1273–1277. [Google Scholar] [CrossRef]
- Prem, B.; Liu, D.T.; Besser, G.; Sharma, G.; Dultinger, L.E.; Hofer, S.V.; Matiasczyk, M.M.; Renner, B.; Mueller, C.A. Long-Lasting Olfactory Dysfunction in COVID-19 Patients. Eur. Arch. Otorhinolaryngol. 2022, 279, 3485–3492. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, M.; Zheng, P.; Shen, W. Residual Olfactory Dysfunction in Coronavirus Disease 2019 Patients after Long Term Recovery. J. Clin. Neurosci. 2021, 93, 31–35. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory Dysfunction and Its Measurement in the Clinic. World J. Otorhinolaryngol. Head Neck Surg. 2015, 1, 28–33. [Google Scholar] [CrossRef]
- Ta, N.H.; Gao, J.; Philpott, C. A Systematic Review to Examine the Relationship between Objective and Patient-reported Outcome Measures in Sinonasal Disorders: Recommendations for Use in Research and Clinical Practice. Int. Forum Allergy Rhinol. 2021, 11, 910–923. [Google Scholar] [CrossRef]
- Biele, G.; Gustavson, K.; Czajkowski, N.O.; Nilsen, R.M.; Reichborn-Kjennerud, T.; Magnus, P.M.; Stoltenberg, C.; Aase, H. Bias from Self Selection and Loss to Follow-up in Prospective Cohort Studies. Eur. J. Epidemiol. 2019, 34, 927–938. [Google Scholar] [CrossRef]
- Du, X.; Wu, G.; Zhu, Y.; Zhang, S. Exploring the Epidemiological Changes of Common Respiratory Viruses since the COVID-19 Pandemic: A Hospital Study in Hangzhou, China. Arch. Virol. 2021, 166, 3085–3092. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.C.; Rossi, Á.D.; Galliez, R.M.; Faffe, D.S.; Tanuri, A.; Castiñeiras, T.M.P.P. Olfactory Dysfunction in Patients with Mild COVID-19 During Gamma, Delta, and Omicron Waves in Rio de Janeiro, Brazil. JAMA 2022, 328, 582. [Google Scholar] [CrossRef] [PubMed]


| Mean age (years) ± SD (Range) | 46.8 ± 13.9 (23–89) | |||||||
| n | % | [95% CI] | ||||||
| Gender | ||||||||
| Male | 32 | 31.4 | [22.5, 41.3] | |||||
| Female | 70 | 68.6 | [58.7, 77.5] | |||||
| Comorbidity | 13 | 12.7 | [7.0, 20.8] | |||||
| Symptoms | During acute COVID-19 infection | 12 months post-COVID-19 infection | McNemar test | |||||
| n | % | [95% CI] | n | % | [95% CI] | χ2 | p | |
| Chemosensory dysfunction | 84 | 82.4 | [73.6, 89.2] | 46 | 45.1 | [35.2, 55.3] | <0.001 | <0.001 |
| Combined smell and taste dysfunction | 70 | 68.6 | [58.7, 77.5] | 27 | 26.5 | [18.2, 36.1] | 0.0018 | <0.001 |
| Isolated smell dysfunction | 8 | 7.8 | [3.4, 14.9] | 16 | 15.7 | [9.2, 24.2] | 0.0054 | 0.0768 |
| Isolated taste dysfunction | 6 | 5.9 | [2.2, 12.4] | 3 | 2.9 | [0.6, 8.4] | 0.0403 | 0.4531 |
| Nasal obstruction | 43 | 42.2 | [32.4, 52.3] | 27 | 26.5 | [18.2, 36.1] | 0.0358 | 0.0149 |
| Nasal discharge | 59 | 57.8 | [47.7, 67.6] | 35 | 34.3 | [25.2, 44.4] | 0.4586 | 0.0011 |
| Facial pain | 33 | 32.4 | [23.4, 42.3] | 14 | 13.7 | [7.7, 22.0] | 0.1286 | 0.0017 |
| Cough | 76 | 74.5 | [64.9, 82.6] | 17 | 16.7 | [10.0, 25.3] | 0.6844 | <0.001 |
| Dyspnea | 56 | 54.9 | [44.7, 64.8] | 26 | 25.5 | [17.4, 35.1] | 0.4308 | <0.001 |
| Fever | 57 | 55.9 | [45.7, 65.7] | 2 | 2.0 | [0.2, 6.9] | 0.1079 | <0.001 |
| Diarrhea | 57 | 55.9 | [45.7, 65.7] | 2 | 2.0 | [0.2, 6.9] | 0.2044 | <0.001 |
| Symptoms | During Acute COVID-19 Infection | 12 Months Post-COVID-19 Infection | |||
|---|---|---|---|---|---|
| ± SE | ± SE | Change ± SE | t | p | |
| Loss of smell | 67.6 ± 42.3 | 15.2 ± 25.9 | 52.4 ± 41.4 | 12.788 | <0.001 |
| Loss of taste | 59.7 ± 40.0 | 9.6 ± 20.7 | 50.2 ± 43.2 | 11.724 | <0.001 |
| Nasal obstruction | 24.2 ± 32.4 | 8.0 ± 17.0 | 16.1 ± 31.6 | 5.149 | <0.001 |
| Nasal discharge | 31.8 ± 32.7 | 11.1 ± 21.3 | 20.7 ± 36.2 | 5.788 | <0.001 |
| Facial pain | 21.6 ± 33.6 | 4.1 ± 12.2 | 17.4 ± 34.2 | 5.146 | <0.001 |
| Cough | 44.1 ± 6.5 | 6.4 ± 16.7 | 37.6 ± 36.5 | 10.412 | <0.001 |
| Dyspnea | 27.1 ± 31.4 | 7.3 ± 16.4 | 19.9 ± 32.2 | 6.228 | <0.001 |
| Intensity of Symptoms during Acute COVID-19 Infection | Intensity of Symptoms 12 Months Post-COVID-19 Infection | |||||
|---|---|---|---|---|---|---|
| Loss of Smell | No | Mild | Moderate | Severe | Total | CI 95% |
| No | 23 | 1 | 0 | 0 | 24 (23.5%) | [15.7, 33.0] |
| Mild | 1 | 2 | 0 | 0 | 3 (2.9%) | [0.6, 8.4] |
| Moderate | 6 | 4 | 1 | 0 | 11 (10.8%) | [5.5, 18.5] |
| Severe | 29 | 20 | 7 | 8 | 64 (62.7%) | [52.6, 72.1] |
| Total | 59 (57.8%) | 27 (26.5%) | 8 (7.8%) | 8 (7.8%) | 102 (100%) | - |
| CI 95% | [47.7, 67.6] | [18.2, 36.1] | [3.4, 14.9] | [3.4, 14.9] | - | - |
| Loss of taste | No | Mild | Moderate | Severe | Total | CI 95% |
| No | 24 | 0 | 1 | 1 | 26 (25.5%) | [17.4, 35.1] |
| Mild | 2 | 0 | 0 | 0 | 2 (2.0%) | [0.2, 6.9] |
| Moderate | 12 | 8 | 1 | 1 | 22 (21.6%) | [14.0, 30.8] |
| Severe | 33 | 11 | 6 | 2 | 52 (51.0%) | [40.9, 61.0] |
| Total | 71 (69.6%) | 19 (18.6%) | 8 (7.8%) | 4 (3.9%) | 102 (100%) | - |
| CI 95% | [59.7, 78.3] | [11.6, 27.6] | [3.4, 14.9] | [1.1, 9.7] | - | - |
| Loss of Smell | Loss of Taste | |||||
|---|---|---|---|---|---|---|
| Time for Recovery | n | % | 95% CI | n | % | 95% CI |
| ≤2 weeks | 18 | 50.0 | [32.9, 67.1] | 27 | 57.4 | [42.2, 71.7] |
| 2 ≤ 4 weeks | 8 | 22.2 | [10.1, 39.2] | 9 | 19.1 | [9.1, 33.3] |
| 4 ≤ 24 weeks | 10 | 27.8 | [14.2, 45.2] | 11 | 23.4 | [12.3, 38.0] |
| 24 < 48 weeks | 0 | 0.0 | [0, 0] | 0 | 0.0 | [0, 0] |
| Total | 36 | 47 | ||||
| Characteristics | Crude OR *1 | 95% CI | p | Adjusted OR *2 | 95% CI | p |
|---|---|---|---|---|---|---|
| Age | ||||||
| ≤40 | ||||||
| >40 | 0.20 | [0.08, 0.52] | 0.010 | 0.20 | [0.07, 0.56] | 0.0023 |
| Fever at the baseline | ||||||
| No | ||||||
| Yes | 3.19 | [1.28, 7.94] | 0.0126 | 2.75 | [1.00, 7.61] | 0.0511 |
| Duration of smell loss | ||||||
| ≤4 weeks | ||||||
| >4 weeks | 0.25 | [0.10, 0.64] | 0.0040 | 0.27 | [0.10, 0.76] | 0.0133 |
| Duration of taste loss | ||||||
| ≤4 weeks | ||||||
| >4 weeks | 0.31 | [0.12, 0.80] | 0.0153 | - | - | - |
| Loss of Smell *1 | Adjusted β ( ± SE) | 95% CI | p | R2 |
|---|---|---|---|---|
| Age | −0.49 ± 0.22 | [−0.93, −0.04] | 0.0327 | 0.5446 |
| Duration of smell loss (weeks) | −0.66 ± 0.23 | [−1.10, −0.21] | <0.0001 | |
| VAS score for loss of smell at baseline | 0.86 ± 0.09 | [0.68, 1.04] | 0.0045 | |
| Loss of Taste *2 | Adjusted β (± SE) | 95% CI | p | R2 |
| Duration of taste loss (weeks) | −0.79 ± 0.19 | [−1.17, −0.42] | <0.0001 | 0.7496 |
| VAS score for loss of taste at baseline | 1.07 ± 0.07 | [0.93, 1.21] | <0.0001 |
| Intensity of Olfactory Loss at the Baseline (Acute COVID-19 Infection) | Intensity of Olfactory Loss 12 Months Post-COVID-19 Infection | |||||
|---|---|---|---|---|---|---|
| No | Mild | Moderate | Severe | Total | CI 95% | |
| No | 1 * | 0 | 0 | 0 | 1 (3.6%) | [0.1, 18.3] |
| Mild | 0 | 1 | 0 | 0 | 1 (3.6%) | [0.1, 18.3] |
| Moderate | 0 | 1 | 1 | 0 | 2 (7.1%) | [0.9, 23.5] |
| Severe | 2 | 12 | 4 | 6 | 24 (85.7%) | [67.3, 96.0] |
| Total | 3 (10.7%) | 14 (50.0%) | 5 (17.9%) | 6 (21.4%) | 28 (100%) | - |
| CI 95% | [2.3, 28.2] | [30.6, 69.4] | [6.1, 36.9] | [8.3, 41.0] | - | - |
| Characteristics | Crude OR *1 | 95% CI | p | Adjusted OR *2 | 95% CI | p |
|---|---|---|---|---|---|---|
| Loss of smell at baseline | ||||||
| ≤30 | ||||||
| >30 | 6.63 | [1.46, 30.23] | 0.0145 | 6.27 | [1.32, 29.87] | 0.0211 |
| Loss of taste at baseline | ||||||
| ≤30 | ||||||
| >30 | 7.04 | [1.55, 32.0] | 0.0116 | |||
| Type of chemosensory dysfunction | ||||||
| Isolated | ||||||
| Combined | 5.37 | [1.49, 19.42] | 0.0104 | |||
| Fever at baseline | ||||||
| No | ||||||
| Yes | 0.40 | [0.16, 0.96] | 0.0407 | |||
| Duration of smell loss | ||||||
| ≤12 weeks | ||||||
| >12 weeks | 4.53 | [1.49, 13.78] | 0.0077 | |||
| Duration of taste loss | ||||||
| ≤12 weeks | ||||||
| >12 weeks | 6.54 | [1.96, 21.82] | 0.0023 | 6.16 | [1.74, 21.86] | 0.0049 |
| Loss of Smell VAS 12-Months Post-COVID-19 Infection | UPSIT Outcome 12-Months Post-COVID-19 Infection | ||||||
|---|---|---|---|---|---|---|---|
| Normosmia | Mild Microsmia | Moderate Microsmia | Severe Microsmia | Anosmia | Total | 95% CI | |
| No | 8 | 13 | 8 | 2 | 1 | 32 (46.4%) | [34.3, 58.8] |
| Mild | 3 | 14 | 3 | 2 | 1 | 23 (33.3%) | [22.4, 45.7] |
| Moderate | 1 | 2 | 2 | 1 | 1 | 7 (10.1%) | [4.2, 19.8] |
| Severe | 0 | 1 | 2 | 2 | 2 | 7 (10.1%) | [4.2, 19.8] |
| Total | 12 (17.4%) | 30 (43.5%) | 15 (21.7%) | 7 (10.1%) | 5 (7.2%) | 69 (100%) | |
| 95% CI | [9.3, 28.4] | [31.6, 56.0] | [12.7, 33.3] | [4.2, 19.8] | [2.4, 16.1] | ||
| UPSIT Scores *1 | Adjusted β ( ± SE) | 95% CI | p | R2 |
|---|---|---|---|---|
| Age | −0.14 ± 0.05 | [−0.23, −0.05] | 0.0042 | 0.2294 |
| VAS score for loss of smell 12 months post-COVID-19 infection | −0.07 ± 0.02 | [−0.12, −0.03] | 0.0034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callejón-Leblic, M.A.; Martín-Jiménez, D.I.; Moreno-Luna, R.; Palacios-Garcia, J.M.; Alvarez-Cendrero, M.; Vizcarra-Melgar, J.A.; Fernandez-Velez, C.; Reyes-Tejero, I.M.; Maza-Solano, J.; Gonzalez-Garcia, J.; et al. Analysis of Prevalence and Predictive Factors of Long-Lasting Olfactory and Gustatory Dysfunction in COVID-19 Patients. Life 2022, 12, 1256. https://doi.org/10.3390/life12081256
Callejón-Leblic MA, Martín-Jiménez DI, Moreno-Luna R, Palacios-Garcia JM, Alvarez-Cendrero M, Vizcarra-Melgar JA, Fernandez-Velez C, Reyes-Tejero IM, Maza-Solano J, Gonzalez-Garcia J, et al. Analysis of Prevalence and Predictive Factors of Long-Lasting Olfactory and Gustatory Dysfunction in COVID-19 Patients. Life. 2022; 12(8):1256. https://doi.org/10.3390/life12081256
Chicago/Turabian StyleCallejón-Leblic, María A., Daniel I. Martín-Jiménez, Ramón Moreno-Luna, Jose M. Palacios-Garcia, Marta Alvarez-Cendrero, Julissa A. Vizcarra-Melgar, Carlos Fernandez-Velez, Isabel M. Reyes-Tejero, Juan Maza-Solano, Jaime Gonzalez-Garcia, and et al. 2022. "Analysis of Prevalence and Predictive Factors of Long-Lasting Olfactory and Gustatory Dysfunction in COVID-19 Patients" Life 12, no. 8: 1256. https://doi.org/10.3390/life12081256