Phytochemical Analysis and Binding Interaction of Cotton Seed Cake Derived Compounds with Target Protein of Meloidogyne incognita for Nematicidal Evaluation
Abstract
:1. Introduction
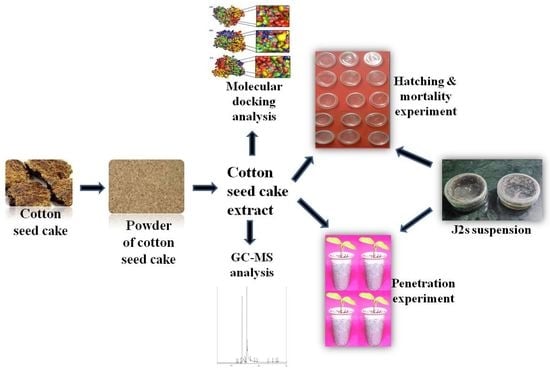
2. Materials and Methods
2.1. Materials
2.2. Collection and Multiplication of Inoculums (J2s) of M. incognita
3. Identification of Meloidogyne Species through Scanning Electron Microscopy (SEM) Analysis
3.1. Preparation of CSC Extract
3.2. GC-MS Analysis of CSC Extract
3.3. Mortality Test
3.4. Hatching Bioassay
3.5. J2 Infection Bioassay
3.6. Molecular Modeling and Docking
Molecular Docking
3.7. Protein and Ligand Preparation
3.8. Statistical Analysis
4. Results and Discussion
4.1. GC-MS Analysis
4.2. Molecular Docking Analysis
4.3. Evaluation of Different Concentrations of CSC on J2s’ Mortality and J2s’ Hatching Inhibition of M. incognita
4.4. Effect of Different Concentrations of CSC on J2s’ Penetration in Roots of Tomato Seedlings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, J.T.; Haegeman, A.; Danchin, E.G.; Gaur, H.S.; Helder, J.; Jones, M.G.K.; Kikuchi, T.; Manzanilla-López, R.; Palomares-Rius, J.E.; Wesemael, W.M.L.; et al. Top 10 plant-parasitic nematodes in molecular plant pathology. Mol. Plant Pathol. 2013, 14, 946–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Singh, B.; Singh, A.P. Nematodes: A threat to sustainability of agriculture. Procedia Environ. Sci. 2015, 29, 215–216. [Google Scholar] [CrossRef] [Green Version]
- Fuller, V.L.; Lilley, C.J.; Urwin, P.E. Nematode resistance. New Phytol. 2008, 180, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Barcala, M.; Cabrera, J.; Fenoll, C. Overview of root-knot nematodes and giant cells. Adv. Bot. Res. 2015, 73, 1–32. [Google Scholar]
- Trudgill, D.L.; Blok, V.C. Apomictic polyphagous root-knot nematodes; exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef]
- Reganold, J.P.; Wachter, J.M. Organic agriculture in the twenty-first century. Nat. Plants. 2016, 2, 15221. [Google Scholar] [CrossRef]
- Bengtsson, J.; Ahnstrom, J.; Weibull, A.C. The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 2010, 42, 261–269. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity a meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, U.N.; Ayres, E.; Wall, D.H.; Li, G.; Bardgett, R.D.; Wu, T.; Garey, J.R. Global-scale patterns of assemblage structure of soil nematodes in relation to climate and ecosystem properties. Glob. Ecol. Biogeogr. 2014, 23, 96–8978. [Google Scholar] [CrossRef]
- Çulcuoğlu, E.; Ünay, E.; Karaosmanoğlu, F. Rapeseed cake as a biomass source. Energy Sources 2002, 24, 329–336. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications—A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Verma, M.; Sharma, A. Utilization of non edible oil seed cakes as substrate for growth of Paecilomyces lilacinus and as biopesticide against termites. Waste Biomass Valorization 2013, 4, 325–330. [Google Scholar] [CrossRef]
- Leggett, M.; Leland, J.; Kellar, K.; Epp, B. Formulation of microbial biocontrol agents—An industrial perspective. Can. J. Plant Pathol. 2011, 33, 101–107. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Wang, K.; Su, L.; Li, H.; Li, R.; Shen, Q. The nematicidal effect of Camellia seed cake on root-knot nematode Meloidogyne javanica of banana. PLoS ONE 2015, 10, e0119700. [Google Scholar] [CrossRef] [Green Version]
- Melo, W.C.; Silva, D.B.; Pereira, N., Jr.; Santa Anna, L.M.M.; Santos, A.S. Producao de etanol a partir de torta de mamona (Ricinuscommunis L.) e avaliacao da letalidade da torta hidrolisada para camundongos. Quim. Nova. 2008, 5, 1104–1106. [Google Scholar] [CrossRef] [Green Version]
- Sumbul, A.; Rizvi, R.; Mahmood, I.; Ansari, R.A. Oil-cake amendments: Useful tools for the management of phytonematodes. Asian J. Plant Pathol. 2015, 9, 91–111. [Google Scholar] [CrossRef] [Green Version]
- VasudhaUdupa, A.; Gowda, B.; Kumarswammy, B.E.; Shivanna, M.B. The antimicrobial and antioxidant property, GC–MS analysis of non-edible oil-seed cakes of neem, madhuca, and simarouba. Bull. Natl. Res. Cent. 2021, 45, 41. [Google Scholar] [CrossRef]
- Pedroso, L.A.; Campos, V.P.; Pedroso, M.P.; Barros, A.F.; Freire, E.S.; Resende, F.M. Volatile organic compounds produced by castor bean cake incorporated into the soil exhibit toxic activity against Meloidogyne Incognita. Pest Manag. Sci. 2019, 75, 476–483. [Google Scholar] [CrossRef]
- Tiyagi, S.A.; Mahmood, I.; Rizvi, R. Application of some latex bearing plants for the management of phytonematodes infecting tomato and eggplant. Thai J. Agric. Sci. 2009, 42, 183–189. [Google Scholar]
- Ashraf, M.S.; Khan, T.A. Integrated approach for the management of Meloidogyne javanica on eggplant using oil cakes and biocontrol agents. Arch. Phytopathol. Plant Prot. 2010, 43, 609–614. [Google Scholar] [CrossRef]
- Radwan, M.A.; El-Maadawy, E.K.; Kassem, S.I.; Abu-Elamayem, M.M. Oil cakes soil amendment effects on Meloidogyne incognita, root-knot nematode infecting tomato. Arch. Phytopathol. Plant Prot. 2009, 42, 58–64. [Google Scholar] [CrossRef]
- Goswami, B.K.; Pandey, R.K.; Rathour, K.S.; Bhattacharya, C.; Singh, L. Integrated application of some compatible biocontrol agents along with mustard oil seed cake and furadan on Meloidogyne incognita infecting tomato plants. J. Zhejiang Univ. Sci. B 2006, 7, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.S.; Tayung, K. Effect of Different Oil Cakes against Root Knot Nematode Infecting Tomato. J. Emerg. Technol. Innov. Res. 2021, 8, 908–918. Available online: https://www.jetir.org/view?paper=JETIR2106686 (accessed on 11 December 2022).
- Sahu, S.; Patra, M.K.; Dash, B. Management of root knot nematode (Meloidogyne incognita) in tomato (cv. Pusa ruby) using different oil cakes. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2527–2532. [Google Scholar] [CrossRef]
- Chen, R.S.; Wang, K.L.; Wu, C.Y. Assessment of the camellia seed meal impact on loaches in paddy fields. Paddy Water Environ. 2012, 10, 291–300. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, M.; Li, Y.; Lin, W.; Hong, S. Effects of Camellia cake organic fertilizer on growth of turf grass Cynodon spp. Rich. J. Cent. South Univ. For. Technol. 2013, 4, 1–6. [Google Scholar]
- Lu, Q.; Liu, T.; Wang, N.; Dou, Z.; Wang, K.; Zuo, Y. Nematicidal effect of methyl palmitate and methyl stearate against Meloidogyne incognita in bananas. J. Agric. Food Chem. 2020, 68, 6502–6510. [Google Scholar] [CrossRef]
- Saha, S.; Walia, S.; Kumar, J.; Parmer, B.S.; Prasad, D. Synergistic/potential interaction between nematostatic constituents from Azadirachta indica, Madhuca indica and Sapindus mukorossi. Arch. Phytopathol. Plant Prot. 2010, 43, 357–367. [Google Scholar] [CrossRef]
- Lee, A.; Kim, D. CRDS: Consensus reverse docking system for target fishing. Bioinformatics 2020, 36, 959–960. [Google Scholar] [CrossRef]
- Bharathi, A.; Roopan, S.M.; Vasavi, C.S.; Munusami, P.; Gayathri, G.A.; Gayathri, M. In silico molecular docking and in vitro antidiabetic studies of dihydropyrimido [4, 5-a] acridin-2-amines. BioMed. Res. Int. 2014, 1, 971569. [Google Scholar] [CrossRef] [Green Version]
- Keerthiraj, M.; Mandal, A.; Dutta, T.K.; Saha, S.; Dutta, A.; Singh, A.; Kundu, A. Nematicidal and Molecular Docking Investigation of Essential Oils from Pogostemon cablin Ecotypes against Meloidogyne Incognita. Chem. Biodivers. 2021, 18, e2100320. [Google Scholar] [CrossRef]
- Kundu, A.; Dutta, A.; Mandal, A.; Negi, L.; Malik, M.; Puramchatwad, R.; Antil, J.; Singh, A.; Rao, U.; Saha, S.; et al. A Comprehensive in vitro and in silico analysis of nematicidal action of essential oils. Front. Plant Sci. 2021, 11, 614143. [Google Scholar] [CrossRef]
- Eisenback, J.D.; Hunt, D.J. General Morphology. In Root-Knot Nematodes; Starr, J.L., Moens, M., Perry, R.N., Eds.; CABI: Wallingford, UK, 2009; pp. 18–54. [Google Scholar]
- Isabel, M.D.O.; Santos, M.S.N.D.A. A technique for preparing perineal patterns of root-knot nematodes for scanning electron microscopy. J Nematol. 1989, 21, 138. [Google Scholar]
- El-Rokiek, K.G.; El-Nagdi, W.M. Dual effects of leaf extracts of Eucalyptus citriodora on controlling purslane and root-knot nematode in sunflower. J. Plant Prot. Res. 2011, 51, 121–129. [Google Scholar] [CrossRef]
- Aissani, N.; Urgeghe, P.P.; Oplos, C.; Saba, M.; Tocco, G.; Petretto, G.L.; Eloh, K.; Menkissoglu-Spiroudi, U.; Ntalli, N.; Caboni, P. Nematicidal activity of the volatilome of Eruca sativa on Meloidogyne incognita. J. Agric. Food Chem. 2015, 63, 6120–6125. [Google Scholar] [CrossRef]
- Sakuma, M. Probit analysis of preference data. Appl. Entomol. Zool. 1998, 33, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Behreus, A.S.; Karbeur, L. Determination of LD50. Archiv. Experiment. Pathol. Pharmakol. 1953, 28, 177–183. [Google Scholar]
- Bridge, J.; Page, S.; Jordan, S. An improved method for staining nematodes in roots. Rothamstead Exp. Stn. Annu. Rep. 1982, 1, 171. [Google Scholar]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDockVina 1.2. 0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Akram, M.; Lal, H.; Shakya, S.; Varshney, R.; Kabir-ud-Din. Molecular engineering of complexation between RNA and biodegradable cationic gemini surfactants: Role of the hydrophobic chain length. Mol. Syst. Des. Eng. 2022, 7, 487–506. [Google Scholar] [CrossRef]
- Studio, D. Dassaults Ystemes BIOVIA, Discovery Studio Modelling Environment, Release 4.5; Accelrys Software Inc.: San Diego, CA, USA, 2015; pp. 98–104. [Google Scholar]
- Sheoran, O.P.; Tonk, D.S.; Kaushik, L.S.; Hasija, R.C.; Pannu, R.S. Statistical Software Package for Agricultural Research Workers; Recent Advances in Information Theory, Statistics & Computer Applications; Hooda, D.S., Hasija, R.C., Eds.; Department of Mathematics Statistics, CCS HAU: Hisar, India, 1998; pp. 139–143. [Google Scholar]
- Huang, Y.; Xu, C.; Ma, L.; Zhang, K.; Duan, C.; Mo, M. Characterization of volatiles produced from Bacillus megaterium YFM3. 25 and their nematicidal activity against Meloidogyne incognita. Eur. J. Plant Pathol. 2010, 126, 417–422. [Google Scholar] [CrossRef]
- Gu, Y.Q.; Mo, M.H.; Zhou, J.P.; Zou, C.S.; Zhang, K.Q. Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol. Biochem. 2007, 39, 2567–2575. [Google Scholar] [CrossRef]
- Monteiro, T.S.A.; Nasu, É.D.G.C.; Guimarães, C.P.; Neves, W.D.S.; Mizobutsi, E.H.; Freitas, L.G.D. Redução de inóculo de Aphelenchoides besseyiems ementes de Brachiaria brizanthatratadas com óleosessenciais. Cienc. Rural 2014, 44, 1149–1154. [Google Scholar] [CrossRef] [Green Version]
- Bell, A.A. Physiology of secondary products. In Cotton Physiology; Mauney, J.R., Stewart, J.M., Eds.; The Cotton Foundation: Memphis, TN, USA, 1986; pp. 597–621. [Google Scholar]
- Opitz, S.; Kunert, G.; Gershenzon, J. Increased terpenoid accumulation in cotton (Gossypium hirsutum) foliage is a general wound response. J. Chem. Ecol. 2008, 34, 508–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenhardt, J.; Köllner, T.G.; Gershenzon, J. Monoterpene and sesquiterpene synthases and the origin of terpene skeletal diversity in plants. Phytochemistry 2009, 70, 1621–1637. [Google Scholar] [CrossRef]
- Jibrin, H.; Gusau, I.A.; Mamman, T.; Musa, M.; Shuaibu, A.A.; Abubakar, M.; Muhammad, U.; Omatah, C.U. Nutritive and phytochemical assessment of cotton (Gossypium spp.) seed meal for fish feed. Int. J. Fish Aquat. Stud. 2020, 8, 380–385. [Google Scholar]
- Qiu, R.; Huang, Z.; Wang, Z. Analysis of fatty acid composition in cottonseed by gas chromatography with on-line pyrolytic methylation. Se Pu Chin. J. Chromatogr. 2018, 36, 925–930. [Google Scholar] [CrossRef]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Ann. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef]
- Ntalli, N.G.; Menksissoglu-Spiroudi, U.; Giannakou, I.O.; Prophetou-Athanasiadou, D.A. Efficacy evaluation of a neem (Azadirachta indica A. Juss) formulation against root-knot nematodes Meloidogyne incognita. Crop Prot. 2009, 28, 489–494. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Seo, D.J.; Kim, K.Y.; Park, R.D.; Kim, D.H.; Han, Y.S.; Kim, T.H.; Jung, W.J. Nematicidal activity of 3,4-dihydroxybenzoic acid purified from Terminalia nigrovenulosa bark against Meloidogyne incognita. Microb. Pathog. 2013, 59, 52–59. [Google Scholar] [CrossRef]
- de Freitas Silva, M.; Campos, V.P.; Barros, A.F.; da Silva, J.C.P.; Pedroso, M.P.; de Jesus Silva, F.; Gomes, V.A.; Justino, J.C. Medicinal plant volatiles applied against the root-knot nematode Meloidogyne incognita. J. Crop Prot. 2020, 130, 105057. [Google Scholar] [CrossRef]
- Kang, J.S.; Moon, Y.S.; Lee, S.H.; Park, I.K. Inhibition of acetylcholinesterase and glutathione S-transferase of the pinewood nematode (Bursaphelenchus xylophilus) by aliphatic compounds. Pestic. Biochem. Physiol. 2013, 105, 184–188. [Google Scholar] [CrossRef]
- Mills, C.; Cleary, B.V.; Walsh, J.J.; Gilmer, J.F. Inhibition of acetylcholinesterase by tea tree oil. J. Pharm. Pharmacol. 2004, 56, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.S.; Lee, D.W.; Koh, Y.H.; Lee, S.H. A soluble acetylcholinesterase provides chemical defense against xenobiotics in the pinewood nematode. PLoS ONE 2011, 6, e19063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, A.O. Biofumigação Do Solo Com Brassica Rapapara o Controle de Fitonematóides; Universidade Federal de Viçosa: Viçosa, Brazil, 2006; p. 44. Available online: http://locus.ufv.br/handle/123456789/4408 (accessed on 11 December 2022).
- Khan, F.; Asif, M.; Khan, A.; Tariq, M.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Evaluation of the nematicidal potential of some botanicals against root-knot nematode, Meloidogyne incognita infected carrot: In vitro and greenhouse study. Curr. Plant Biol. 2019, 20, 100115. [Google Scholar] [CrossRef]
- Konstantopoulou, I.; Vassilopoulou, L.; Mavragani-Tsipidou, P.; Scouras, Z.G. Insecticidal effects of essential oils. A study of the effects of essential oils extracted from eleven Greek aromatic plants on Drosophila auraria. Experientia 1992, 48, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Westcott, S.W., III; Kluepfel, D.A. Inhibition of Criconemellax enoplax egg hatch by Pseudomonas aureofaciens. Phytopathology 1993, 83, 1245. [Google Scholar] [CrossRef]
- El-Habashy, D.E.; Rasoul, M.A.A.; Abdelgaleil, S.A.M. Nematicidal activity of phytochemicals and their potential use for the control of Meloidogyne javanica infected eggplant in the greenhouse. Eur. J. Plant Pathol. 2020, 158, 381–390. [Google Scholar] [CrossRef]
- Sousa, A.J.S.; Souza, P.F.N.; Gifoni, J.M.; Dias, L.P.; Freitas, C.D.T.; Oliveira, J.T.A.; Sousa, D.O.B.; Vasconcelos, I.M. Scanning electron microscopy reveals deleterious effects of Moringa oleifera seed exuded proteins on root-knot nematode Meloidogyne incognita eggs. Internat. J. Biol. Macromol. 2020, 154, 1237–1244. [Google Scholar] [CrossRef]
- Jardim, I.N.; Oliveira, D.F.; Silva, G.H.; Campos, V.P.; Souza, P.E. (E)-cinnamaldehyde from the essential oil of Cinnamomum cassia controls Meloidogyne incognita in soybean plants. J. Pest. Sci. 2018, 91, 479–487. [Google Scholar] [CrossRef]
- Ismail, A.E.; Abd-El-Migeed, M.M.; Azza, R.A.; Awaad, M.S. Meloidogyne incognita suppression and changes of grapevine yield properties determined by waste residues from jojoba, black seed oil extraction and slow release of nitrogen fertilizer. Pak. J. Nematol. 2011, 29, 67–85. [Google Scholar]
- Khan, A.M.; Alam, M.M.; Ahmad, R. Mechanism of the control of plant-parasitic nematodes as a result of the application of oilcakes. Indian J. Nematol. 1974, 4, 93–96. [Google Scholar]
- Mojtahedi, H.; Santo, G.S.; Wilson, J.H.; Hang, A.N. Managing Meloidogyne chitwoodi on potato with rapeseed as green manure. Plant Dis. 1993, 77, 42–46. [Google Scholar] [CrossRef]
- Aoudia, H.; Ntalli, N.; Aissani, R.N.; Zaidi, Y.; Caboni, P. Nematotoxic phenolic compounds from Melia azedarach against Meloidogyne incognita. J. Agric. Food Chem. 2012, 60, 11675–11680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ruan, W.; Deng, Y.; Gao, Y. Potential antagonistic effects of nine natural fatty acids against Meloidogyne incognita. J. Agric. Food Chem. 2012, 60, 11631–11637. [Google Scholar] [CrossRef]
- Dammini-Premachandra, W.T.C.; Mampitiyarachchi, H.; Ebssa, L. Nemato-toxic potential of Betel (Piper betle L.) (Piperaceae) leaf. J. Crop Prot. 2014, 65, 1–5. [Google Scholar] [CrossRef]
- Baldwin, J.G.; Bell, A.H. Pararotylenchus n. gen. (Pararotylenchinae n. subfam., Hoplolaimidae) with six new species and two new combinations. J. Nematol. 1981, 13, 111. [Google Scholar]
- Oka, Y.; Gözel, U.; Spiegel, Y.; Mor, M. Cereal cyst nematodes in Israel, and their biology and control strategies. In Cereal Cyst Nematodes: Status, Research and Outlook, Proceedings of the First Workshop of the International Cereal Cyst Nematode Initiative, Antalya, Turkey, 21–23 October 2009; International Maize and Wheat Improvement Centre (CIMMYT): Mexico City, Mexico, 2009; pp. 118–123. [Google Scholar]
- Ulfa, M.; Himawan, T.; Tarno, H. Nematicidal activity of turmeric extract against nematodes Meloidogyne spp. Res. J. Life Sci. 2021, 8, 48–56. [Google Scholar] [CrossRef]





| Peak Number | Retention Time | Area | Area% | Name of Compound | Molecular Weight | Molecular Formula |
|---|---|---|---|---|---|---|
| 1 | 12.024 | 1,906,993 | 0.41 | Tetradecanoic acid | 228.37 | C14H28O2 |
| 2 | 12.467 | 114,218 | 0.02 | Neophytadiene | 278.5 | C20H38 |
| 3 | 13.806 | 2,301,736 | 0.49 | Phthalic acid | 166.13 | C8H6O4 |
| 4 | 14.000 | 170,834,132 | 36.40 | n-Hexadecanoic acid | 256.42 | C16H32O2 |
| 5 | 15.313 | 282,292 | 0.06 | Methyl stearate | 298.5 | C19H38O2 |
| 6 | 15.683 | 283,687,872 | 60.45 | 9,12-Octadecadienoic acid | 280.4 | C18H32O2 |
| 7 | 15.832 | 5,263,067 | 1.12 | 9-Octadecenoic acid | 282.5 | C18H34O2 |
| 8 | 16.988 | 139,234 | 0.03 | Hexadecanal | 240.42 | C16H32O |
| 9 | 18.503 | 640,704 | 0.14 | Octadecanoic acid | 284.47 | C18H36O2 |
| 10 | 23.410 | 179,662 | 0.04 | Squalene | 410.7 | C30H50 |
| 11 | 26.878 | 1,276,644 | 0.27 | Stigmast-5-en-3-ol | 414.7 | C29H50O |
| Ligands | Binding Free Energy (kcal/mol) | Interactions | |
|---|---|---|---|
| Hydrogen Bonding | Others | ||
| 9,12-Octadecadienoic acid | −5.3 | Asn283, Thr287, and Asn363 | Ile282, Asn284, Ala286, Phe365, Leu601, Glu602, Gln605, and Ala606 |
| n-Hexadecanoic acid | −4.5 | - | Asp126, Met128, Tyr173, Asp336, Ser339, Pro345, Met346 Asp347, Phe348, Ala349, Trp391, and Tyr394 |
| Tetradecanoic acid | −4.9 | Thr287 and Asn363 | Ile282, Asn284, Lys288, Pro289, Asp354, Phe365, Por432, Tyr433, and Phe434 |
| Treatment | Exposure Period (Hours) | LC 50 Value in mg/L (95% CL) |
|---|---|---|
| Cotton seed cake | 12 | 3362.85 |
| 24 | 1749.67 | |
| 36 | 873.05 | |
| 48 | 462.95 |
| Treatment | Concentration (mg/L) | Number of Dead J2s (Mean ± SE) at Different Time Intervals (Hours) | |||
|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | ||
| Cotton seed cake | 250 | 8 c ± 1.53 (8.89%) | 13 c ± 1.53 (14.44%) | 19 d ± 1.53 (21.11%) | 30 c ± 2.08 (33.33%) |
| 500 | 12 c ± 2.08 (13.33%) | 18 c ± 2.52 (20.00%) | 30 c ± 2.31 (33.33%) | 47 b ± 2.08 (52.22%) | |
| 750 | 17 b ± 1.53 (18.89%) | 25 b ± 2.31 (27.78%) | 39 b ± 2.31 (43.33%) | 54 b ± 1.53 (60.00%) | |
| 1000 | 25 a ± 2.08 (27.78%) | 37 a ± 1.73 (41.11%) | 51 a ± 3.21 (56.67%) | 67 a ± 2.52 (74.44%) | |
| DW | 0 d ± 0 (0.00%) | 0 d ± 0 (0.00%) | 0 e ± 0 (0.00%) | 0 d ± 0 (0.00%) | |
| Df | 4 | 4 | 4 | 4 | |
| Sum of Squares | 1059.60 | 2271.60 | 4556.40 | 8019.60 | |
| Mean Squares | 264.90 | 567.90 | 1139.10 | 2004.90 | |
| F-Calculated | 36.79 | 74.72 | 81.36 | 123.75 | |
| Significance | 0.00001 | 0.0000 | 0.0003 | 0.0002 | |
| Treatment | Concentrations (mg/L) | Number of Penetrated J2s (Mean ± SE) at Different Time Intervals (Days) | |
|---|---|---|---|
| 3 | 5 | ||
| Cotton seed cake | 250 | 48 a ± 2.08 (40.00%) | 54 a ± 2.52 (32.50%) |
| 500 | 39 b ± 2.08 (51.25%) | 45 b ± 2.65 (43.75%) | |
| 750 | 30 c ± 2.31 (62.50%) | 37 bc ± 2.08 (53.75%) | |
| 1000 | 25 d ± 1.53 (68.75%) | 29 c ± 1.73 (63.75%) | |
| Df | 3 | 3 | |
| Sum of Squares | 927 | 1034.25 | |
| Mean Squares | 309 | 344.75 | |
| F-Calculated | 15.84 | 18.89 | |
| Significance | 0.001 | 0.0005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, F.M.; Khan, A.; Ajmal, M.R.; Khan, R.H.; Khan, M.F.; Lal, H.; Ullah, M.F.; Ahmad, F.; Ahamad, L.; Khan, A.; et al. Phytochemical Analysis and Binding Interaction of Cotton Seed Cake Derived Compounds with Target Protein of Meloidogyne incognita for Nematicidal Evaluation. Life 2022, 12, 2109. https://doi.org/10.3390/life12122109
Almutairi FM, Khan A, Ajmal MR, Khan RH, Khan MF, Lal H, Ullah MF, Ahmad F, Ahamad L, Khan A, et al. Phytochemical Analysis and Binding Interaction of Cotton Seed Cake Derived Compounds with Target Protein of Meloidogyne incognita for Nematicidal Evaluation. Life. 2022; 12(12):2109. https://doi.org/10.3390/life12122109
Chicago/Turabian StyleAlmutairi, Fahad M., Amir Khan, Mohammad Rehan Ajmal, Rizwan Hasan Khan, Mohd Farhan Khan, Hira Lal, Mohammad Fahad Ullah, Faheem Ahmad, Lukman Ahamad, Arshad Khan, and et al. 2022. "Phytochemical Analysis and Binding Interaction of Cotton Seed Cake Derived Compounds with Target Protein of Meloidogyne incognita for Nematicidal Evaluation" Life 12, no. 12: 2109. https://doi.org/10.3390/life12122109