Red Light Enhances Plant Adaptation to Spaceflight and Mars g-Levels
Abstract
:Abstract
1. Introduction
2. Altered Gravity/Microgravity Disturbs Plant Growth
3. However, Plants May Overcome Gravitational Stress and Adapt to the Space Environment
4. Plant Culture in Space May Benefit from Other Environmental Cues Replacing Gravity
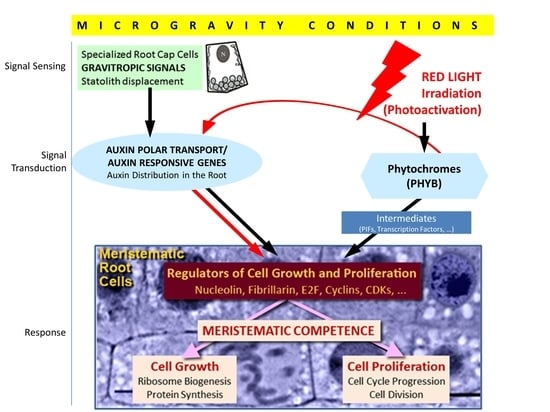
5. The Advantages of Red Light Illumination to Improve Seedling Viability in Space, as Revealed by the SEEDLING GROWTH Experiments
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NASA; CSA; ESA; JAXA; ROSCOSMOS; ASI. International Space Station. Benefits for Humanity, 3rd ed.; NASA NP-2018-06-013-JSC; Robinson, J., Costello, K., Eds.; NASA: Washington, DC, USA, 2018.
- Rinaldi, A. Research in space: In search of meaning. EMBO Rep. 2016, 17, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Voosen, P. NASA Curiosity rover hits organic pay dirt on Mars. Science 2018, 360, 1054–1055. [Google Scholar] [CrossRef] [PubMed]
- Häder, D.-P.; Braun, M.; Hemmersbach, R. Bioregenerative Life Support Systems in space research. In Gravitational Biology I: Gravity Sensing and Graviorientation in Microorganisms and Plants; Ruyters, G., Braun, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 113–122. [Google Scholar]
- Lasseur, C.; Brunet, J.; de Weever, H.; Dixon, M.; Dussap, G.; Godia, F.; Leys, N.; Mergeay, M.; Van Der Straeten, D. MELiSSA: The European project of closed life support system. Gravit. Space Biol. 2010, 23, 3–12. [Google Scholar]
- Wheeler, R.M. Agriculture for space: People and places paving the way. Open Agric. 2017, 2, 14. [Google Scholar] [CrossRef]
- Medina, F.J. Space explorers need to be space farmers: What we know and what we need to know about plant growth in space. Mètode Sci. Stud. J.-Annu. Rev. 2021, 11, 55–62. [Google Scholar] [CrossRef]
- Wamelink, G.W.; Frissel, J.Y.; Krijnen, W.H.; Verwoert, M.R.; Goedhart, P.W. Can plants grow on Mars and the moon: A growth experiment on Mars and moon soil simulants. PLoS ONE 2014, 9, e103138. [Google Scholar] [CrossRef]
- Paul, A.-L.; Elardo, S.M.; Ferl, R. Plants grown in Apollo lunar regolith present stress-associated transcriptomes that inform prospects for lunar exploration. Commun. Biol. 2022, 5, 382. [Google Scholar] [CrossRef] [PubMed]
- Beischer, D.E.; Fregly, A.R. Animals and Man in Space: A Chronology and Annotated Bibliography, through the Year 1960; US Naval School of Aviation Medicine, US Naval Aviation Medical Center: Atlanta, GA, USA, 1962; Volume 5. [Google Scholar]
- Harvey, B.; Zakutnyaya, O. Russian Space Probes: Scientific Discoveries and Future Missions; Springer Science & Business Media/Praxis Publishing: Chichester, UK, 2011. [Google Scholar]
- Zabel, P.; Bamsey, M.; Schubert, D.; Tajmar, M. Review and analysis of over 40 years of space plant growth systems. Life Sci. Space Res. 2016, 10, 1–16. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z. Space, the final frontier: A critical review of recent experiments performed in microgravity. Plant Sci. 2016, 243, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.Z.; Wolverton, C.; Wyatt, S.E.; Hasenstein, K.H.; van Loon, J.J.W.A. Comparison of microgravity analogs to spaceflight in studies of plant growth and development. Front. Plant Sci. 2019, 10, 1577. [Google Scholar] [CrossRef] [PubMed]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; de Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medina, F.J.; Herranz, R.; Arena, C.; Aronne, G.; De Micco, V. Growing plants under generated extra-terrestrial environments: Effects of altered gravity and radiation. In Generation and Applications of Extra-Terrestrial Environments on Earth; Beysens, D., Van Loon, J.J.W.A., Eds.; River Publishers: Delft, The Netherlands, 2015; pp. 239–254. [Google Scholar]
- Van Loon, J.J.W.A. Centrifuges for microgravity simulation. The reduced gravity paradigm. Front. Astron. Space Sci. 2016, 3, 21. [Google Scholar] [CrossRef]
- Schmidt, W. Gravity induced absorption changes in Phycomyces blakesleanus and coleoptiles of Zea mays as measured on the Drop Tower in Bremen (FRG). Microgravity Sci. Technol. 2010, 22, 79–85. [Google Scholar] [CrossRef]
- Pletser, V. European aircraft parabolic flights for microgravity research, applications and exploration: A review. Reach 2016, 1, 11–19. [Google Scholar] [CrossRef]
- Hauslage, J.; Görög, M.; Krause, L.; Schüler, O.; Schäfer, M.; Witten, A.; Kesseler, L.; Böhmer, M.; Hemmersbach, R. ARABIDOMICS—A new experimental platform for molecular analyses of plants in drop towers, on parabolic flights, and sounding rockets. Rev. Sci. Instrum. 2020, 91, 034504. [Google Scholar] [CrossRef]
- Merkys, A.J.; Laurinavicius, R.S.; Svegzdiene, D.V. Plant growth, development and embryogenesis during Salyut-7 flight. Adv Space Res. 1984, 4, 55–63. [Google Scholar] [CrossRef]
- Halstead, T.W.; Dutcher, F.R. Plants in space. Annu. Rev. Plant Physiol. 1987, 38, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Manzano, A.; Carnero-Diaz, E.; Herranz, R.; Medina, F.J. Recent transcriptomic studies to elucidate the plant adaptive response to spaceflight and to simulated space environments. iScience 2022, 25, 104687. [Google Scholar] [CrossRef]
- Tixador, R.; Raffin, J.; Richoilley, G.; Kordium, V.A.; Kojarinov, V.; Maneko, G. Ampoule de verre cassable contenant un liquide sous pression, éjectable en totalité‚ lors de la cassure de l’ampoule. Brevet B. 148 No. 8007471. Innov. Tech. Biol. Med. 1981, 2, 12–14. [Google Scholar]
- Brinckmann, E.; Brillouet, C. Biorack on Spacehab, SP-1222; ESA Publication Division ESTEC: Noordwijk, The Netherlands, 1999. [Google Scholar]
- Matía, I.; González-Camacho, F.; Marco, R.; Kiss, J.Z.; Gasset, G.; Medina, F.J. Nucleolar structure and proliferation activity of Arabidopsis root cells from seedlings germinated on the International Space Station. Adv. Space Res. 2005, 36, 1244–1253. [Google Scholar] [CrossRef]
- Brinckmann, E. ESA hardware for plant research on the International Space Station. Adv. Space Res. 2005, 36, 1162–1166. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Kumar, P.; Millar, K.D.L.; Edelmann, R.E.; Correll, M.J. Operations of a spaceflight experiment to investigate plant tropisms. Adv. Space Res. 2009, 44, 879–886. [Google Scholar] [CrossRef]
- Kittang, A.-I.; Iversen, T.-H.; Fossum, K.R.; Mazars, C.; Carnero-Diaz, E.; Boucheron-Dubuisson, E.; Le Disquet, I.; Legué, V.; Herranz, R.; Pereda-Loth, V.; et al. Exploration of plant growth and development using the European Modular Cultivation System facility on the International Space Station. Plant Biol. 2014, 16, 528–538. [Google Scholar] [CrossRef]
- Bizet, F.; Pereda-Loth, V.; Chauvet, H.; Gérard, J.; Eche, B.; Girousse, C.; Courtade, M.; Perbal, G.; Legué, V. Both gravistimulation onset and removal trigger an increase of cytoplasmic free calcium in statocytes of roots grown in microgravity. Sci. Rep. 2018, 8, 11442. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.-L.; Zupanska, A.K.; Ostrow, D.T.; Zhang, Y.; Sun, Y.; Li, J.-L.; Shanker, S.; Farmerie, W.G.; Amalfitano, C.E.; Ferl, R.J. Spaceflight transcriptomes: Unique responses to a novel environment. Astrobiology 2012, 12, 40–56. [Google Scholar] [CrossRef]
- Paul, A.-L.; Zupanska, A.; Schultz, E.; Ferl, R. Organ-specific remodeling of the Arabidopsis transcriptome in response to spaceflight. BMC Plant Biol. 2013, 13, 112. [Google Scholar] [CrossRef]
- Califar, B.; Sng, N.J.; Zupanska, A.; Paul, A.-L.; Ferl, R.J. Root skewing-associated genes impact the spaceflight response of Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 239. [Google Scholar] [CrossRef]
- Yano, S.; Kasahara, H.; Masuda, D.; Tanigaki, F.; Shimazu, T.; Suzuki, H.; Karahara, I.; Soga, K.; Hoson, T.; Tayama, I.; et al. Improvements in and actual performance of the Plant Experiment Unit onboard Kibo, the Japanese experiment module on the international space station. Adv. Space Res. 2013, 51, 780–788. [Google Scholar] [CrossRef]
- Matía, I.; González-Camacho, F.; Herranz, R.; Kiss, J.Z.; Gasset, G.; van Loon, J.J.W.A.; Marco, R.; Medina, F.J. Plant cell proliferation and growth are altered by microgravity conditions in spaceflight. J. Plant Physiol. 2010, 167, 184–193. [Google Scholar] [CrossRef]
- Mizukami, Y. A matter of size: Developmental control of organ size in plants. Curr. Opin. Plant Biol. 2001, 4, 533–539. [Google Scholar] [CrossRef]
- Manzano, A.I.; Larkin, O.; Dijkstra, C.; Anthony, P.; Davey, M.; Eaves, L.; Hill, R.; Herranz, R.; Medina, F.J. Meristematic cell proliferation and ribosome biogenesis are decoupled in diamagnetically levitated Arabidopsis seedlings. BMC Plant Biol. 2013, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Boucheron-Dubuisson, E.; Manzano, A.I.; Le Disquet, I.; Matía, I.; Sáez-Vasquez, J.; van Loon, J.J.W.A.; Herranz, R.; Carnero-Diaz, E.; Medina, F.J. Functional alterations of root meristematic cells of Arabidopsis thaliana induced by a simulated microgravity environment. J. Plant Physiol. 2016, 207, 30–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal, K.Y.; Herranz, R.; van Loon, J.J.W.A.; Medina, F.J. Cell cycle acceleration and changes in essential nuclear functions induced by simulated microgravity in a synchronized Arabidopsis cell culture. Plant Cell Environ. 2019, 42, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Kamal, K.Y.; van Loon, J.J.W.A.; Medina, F.J.; Herranz, R. Differential transcriptional profile through cell cycle progression in Arabidopsis cultures under simulated microgravity. Genomics 2019, 111, 1956–1965. [Google Scholar] [CrossRef]
- Kamal, K.Y.; Herranz, R.; van Loon, J.J.W.A.; Medina, F.J. Simulated microgravity, Mars gravity, and 2g hypergravity affect cell cycle regulation, ribosome biogenesis, and epigenetics in Arabidopsis cell cultures. Sci. Rep. 2018, 8, 6424. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.J.; Herranz, R. Microgravity environment uncouples cell growth and cell proliferation in root meristematic cells: The mediator role of auxin. Plant Signal. Behav. 2010, 5, 176–179. [Google Scholar] [CrossRef]
- Perrot-Rechenmann, C. Cellular responses to auxin: Division versus expansion. Cold Spring Harb. Perspect. Biol. 2010, 2, a001446. [Google Scholar] [CrossRef]
- David, K.M.; Couch, D.; Braun, N.; Brown, S.; Grosclaude, J.; Perrot-Rechenmann, C. The auxin-binding protein 1 is essential for the control of cell cycle. Plant J. 2007, 50, 197–206. [Google Scholar] [CrossRef]
- Muday, G.K.; Murphy, A.S. An emerging model of auxin transport regulation. Plant Cell 2002, 14, 293–299. [Google Scholar] [CrossRef]
- Millar, K.D.L.; Kumar, P.; Correll, M.J.; Mullen, J.L.; Hangarter, R.P.; Edelmann, R.E.; Kiss, J.Z. A novel phototropic response to red light is revealed in microgravity. New Phytol. 2010, 186, 648–656. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Millar, K.D.L.; Edelmann, R.E. Phototropism of Arabidopsis thaliana in microgravity and fractional gravity on the International Space Station. Planta 2012, 236, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Correll, M.J.; Pyle, T.P.; Millar, K.D.L.; Sun, Y.; Yao, J.; Edelmann, R.E.; Kiss, J.Z. Transcriptome analyses of Arabidopsis thaliana seedlings grown in space: Implications for gravity-responsive genes. Planta 2013, 238, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Zupanska, A.K.; LeFrois, C.; Ferl, R.J.; Paul, A.-L. HSFA2 functions in the physiological adaptation of undifferentiated plant cells to spaceflight. Int. J. Mol. Sci. 2019, 20, 390. [Google Scholar] [CrossRef]
- Schüler, O.; Hemmersbach, R.; Böhmer, M. A bird’s-eye view of molecular changes in plant gravitropism using omics techniques. Front. Plant Sci. 2015, 6, 1176. [Google Scholar] [CrossRef] [PubMed]
- Angelos, E.; Kwan Ko, D.; Zemelis-Durfee, S.; Brandizzi, F. Relevance of the unfolded protein response to spaceflight-induced transcriptional reprogramming in Arabidopsis. Astrobiology 2021, 21, 367–380. [Google Scholar] [CrossRef]
- Kruse, C.P.S.; Meyers, A.D.; Basu, P.; Hutchinson, S.; Luesse, D.R.; Wyatt, S.E. Spaceflight induces novel regulatory responses in Arabidopsis seedling as revealed by combined proteomic and transcriptomic analyses. BMC Plant Biol. 2020, 20, 237. [Google Scholar] [CrossRef]
- Johnson, C.M.; Subramanian, A.; Pattathil, S.; Correll, M.J.; Kiss, J.Z. Comparative transcriptomics indicate changes in cell wall organization and stress response in seedlings during spaceflight. Am. J. Bot. 2017, 104, 1219–1231. [Google Scholar] [CrossRef]
- Khodadad, C.L.M.; Hummerick, M.E.; Spencer, L.E.; Dixit, A.R.; Richards, J.T.; Romeyn, M.W.; Smith, T.M.; Wheeler, R.M.; Massa, G.D. Microbiological and nutritional analysis of lettuce crops grown on the International Space Station. Front. Plant Sci. 2020, 11, 199. [Google Scholar] [CrossRef]
- Link, B.M.; Busse, J.S.; Stankovic, B. Seed-to-Seed-to-Seed growth and development of Arabidopsis in microgravity. Astrobiology 2014, 14, 866–875. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Kiss, J.Z.; Herranz, R.; Medina, F.J. Light and gravity signals synergize in modulating plant development. Front. Plant Sci. 2014, 5, 563. [Google Scholar] [CrossRef]
- Reichler, S.A.; Balk, J.; Brown, M.E.; Woodruff, K.; Clark, G.B.; Roux, S.J. Light differentially regulates cell division and the mRNA abundance of pea nucleolin during de-etiolation. Plant Physiol. 2001, 125, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Bourbousse, C.; Barneche, F.; Laloi, C. Plant chromatin catches the Sun. Front. Plant Sci. 2020, 10, 1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzano, A.; Pereda-Loth, V.; de Bures, A.; Sáez-Vásquez, J.; Herranz, R.; Medina, F.J. Light signals counteract alterations caused by simulated microgravity in proliferating plant cells. Am. J. Bot. 2021, 108, 1775–1792. [Google Scholar] [CrossRef] [PubMed]
- Villacampa, A.; Fañanás-Pueyo, I.; Medina, F.J.; Ciska, M. Root growth direction in simulated microgravity is modulated by a light avoidance mechanism mediated by flavonols. Physiol. Plant. 2022, 174, e13722. [Google Scholar] [CrossRef]
- Manzano, A.; Creus, E.; Tomás, A.; Valbuena, M.A.; Villacampa, A.; Ciska, M.; Edelmann, R.E.; Kiss, J.Z.; Medina, F.J.; Herranz, R. The FixBox: Hardware to provide on-orbit fixation capabilities to the EMCS on the ISS. Microgravity Sci. Technol. 2020, 32, 1105–1120. [Google Scholar] [CrossRef]
- Brunoud, G.; Wells, D.M.; Oliva, M.; Larrieu, A.; Mirabet, V.; Burrow, A.H.; Beeckman, T.; Kepinski, S.; Traas, J.; Bennett, M.J.; et al. A novel sensor to map auxin response and distribution at high spatio-temporal resolution. Nature 2012, 482, 103–106. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Herranz, R.; Medina, F.J.; Edelmann, R.E.; Kiss, J.Z. A novel blue-light phototropic response is revealed in roots of Arabidopsis thaliana in microgravity. Planta 2016, 244, 1201–1215. [Google Scholar] [CrossRef]
- Briggs, W.R. Phototropism: Some history, some puzzles, and a look ahead. Plant Physiol. 2014, 164, 13–23. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Herranz, R.; Poehlman, W.L.; Alex Feltus, F.; Villacampa, A.; Ciska, M.; Medina, F.J.; Kiss, J.Z. RNA-seq analyses of Arabidopsis thaliana seedlings after exposure to blue-light phototropic stimuli in microgravity. Am. J. Bot. 2019, 106, 1466–1476. [Google Scholar] [CrossRef]
- Herranz, R.; Vandenbrink, J.P.; Villacampa, A.; Manzano, A.; Poehlman, W.L.; Feltus, F.A.; Kiss, J.Z.; Medina, F.J. RNAseq analysis of the response of Arabidopsis thaliana to fractional gravity under blue-light stimulation during spaceflight. Front. Plant Sci. 2019, 10, 1529. [Google Scholar] [CrossRef]
- Valbuena, M.A.; Manzano, A.; Vandenbrink, J.P.; Pereda-Loth, V.; Carnero-Diaz, E.; Edelmann, R.E.; Kiss, J.Z.; Herranz, R.; Medina, F.J. The combined effects of real or simulated microgravity and red-light photoactivation on plant root meristematic cells. Planta 2018, 248, 691–704. [Google Scholar] [CrossRef] [PubMed]
- Eichler, G.S.; Huang, S.; Ingber, D.E. Gene Expression Dynamics Inspector (GEDI): For integrative analysis of expression profiles. Bioinformatics 2003, 19, 2321–2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontvianne, F.; Matia, I.; Douet, J.; Tourmente, S.; Medina, F.J.; Echeverria, M.; Saez-Vasquez, J. Characterization of AtNUC-L1 reveals a central role of nucleolin in plant development and nucleolus organization. Proc. FEBS J. 2007, 2007, 74. [Google Scholar]
- Durut, N.; Abou-Ellail, M.; Comella, P.; Jobet, E.; de Bures, A.; Sáez-Vásquez, J. NUCLEOLIN: Similar and antagonistic roles in Arabidopsis thaliana. In Proceedings of the 26th European Low Gravity Research Association Biennial Symposium and General Assembly, Granada, Spain, 24–27 September 2019; p. 150. Available online: https://www.elgra.org/wp-content/uploads/2020/06/ELGRA2019-Book-of-abstracts.pdf (accessed on 1 July 2022).
- Manzano, A.; Villacampa, A.; Sáez-Vásquez, J.; Kiss, J.Z.; Medina, F.J.; Herranz, R. The importance of Earth reference controls in spaceflight -omics research: Characterization of nucleolin mutants from the Seedling Growth experiments. iScience 2020, 23, 101686. [Google Scholar] [CrossRef]
- Herranz, R.; Valbuena, M.A.; Youssef, K.; Medina, F.J. Mechanisms of disruption of meristematic competence by microgravity in Arabidopsis seedlings. Plant Signal. Behav. 2014, 9, e28289. [Google Scholar] [CrossRef]
- Medina, F.J.; Manzano, A.; Villacampa, A.; Ciska, M.; Herranz, R. Understanding reduced gravity effects on early plant development before attempting life-support farming in the Moon and Mars. Front. Astron. Space Sci. 2021, 8, 729154. [Google Scholar] [CrossRef]
- Villacampa, A.; Ciska, M.; Manzano, A.; Vandenbrink, J.P.; Kiss, J.Z.; Herranz, R.; Medina, F.J. From spaceflight to Mars g-levels: Adaptive response of A. thaliana seedlings in a reduced gravity environment is enhanced by red-light photostimulation. Int. J. Mol. Sci. 2021, 22, 899. [Google Scholar] [CrossRef]
- Medina, F.J.; Manzano, A.; Kamal, K.Y.; Ciska, M.; Herranz, R. Plants in space: Novel physiological challenges and adaptation mechanisms. In Progress in Botany; Lüttge, U., Cánovas, F.M., Pretzsch, H., Risueño, M.C., Eds.; Springer Nature: Berlin, Heidelberg, 2021; Volume 83, pp. 1–36. [Google Scholar]
- Manzano, A.I.; Herranz, R.; Manzano, A.; Van Loon, J.J.W.A.; Medina, F.J. Early effects of altered gravity environments on plant cell growth and cell proliferation: Characterization of morphofunctional nucleolar types in an Arabidopsis cell culture system. Front. Astron. Space Sci. 2016, 3, 2. [Google Scholar] [CrossRef]
- Ferl, R.J.; Paul, A.-L. The effect of spaceflight on the gravity-sensing auxin gradient of roots: GFP reporter gene microscopy on orbit. NPJ Microgravity 2016, 2, 15023. [Google Scholar] [CrossRef]
- Bräutigam, K.; Dietzel, L.; Pfannschmidt, T. Plastid-nucleus communication: Anterograde and retrograde signalling inthe development and function of plastids. In Cell and Molecular Biology of Plastids; Bock, R., Ed.; Springer: Berlin, Heidelberg, 2007; pp. 409–455. [Google Scholar]
- Phukan, U.J.; Jeena, G.S.; Shukla, R.K. WRKY transcription factors: Molecular regulation and stress responses in plants. Front. Plant Sci. 2016, 7, 760. [Google Scholar] [CrossRef]
- Van Aken, O.; Zhang, B.; Law, S.; Narsai, R.; Whelan, J. AtWRKY40 and AtWRKY63 modulate the expression of stress-responsive nuclear genes encoding mitochondrial and chloroplast proteins. Plant Physiol. 2013, 162, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina, F.-J.; Manzano, A.; Herranz, R.; Kiss, J.Z. Red Light Enhances Plant Adaptation to Spaceflight and Mars g-Levels. Life 2022, 12, 1484. https://doi.org/10.3390/life12101484
Medina F-J, Manzano A, Herranz R, Kiss JZ. Red Light Enhances Plant Adaptation to Spaceflight and Mars g-Levels. Life. 2022; 12(10):1484. https://doi.org/10.3390/life12101484
Chicago/Turabian StyleMedina, Francisco-Javier, Aránzazu Manzano, Raúl Herranz, and John Z. Kiss. 2022. "Red Light Enhances Plant Adaptation to Spaceflight and Mars g-Levels" Life 12, no. 10: 1484. https://doi.org/10.3390/life12101484