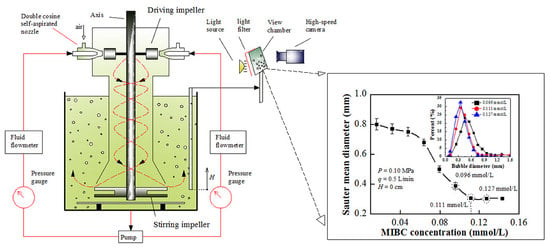
1. Introduction
Flotation devices are considered to be effective for fine coal slime separation processes [
1,
2], and the research and development of flotation equipment are focused on achieving a large-scale, high-efficiency, energy-saving, and environmentally friendly separation [
3,
4]. To date, three main types of flotation machines have been widely used in different flotation process of minerals: mechanical flotation machine [
5], Jameson cell [
6], and flotation column [
7].
Extensive studies on the suction capacity and bubble size distribution (BSD) of traditional mechanical flotation machines have indicated that these functions are easily influenced by the structural parameters of an “impeller stator” [
8], which has higher energy consumption due to mechanical agitation [
9] and larger bubbles, compared to nanobubble column flotation [
10]. For the Jameson cell, air in the suction mode is continuously pumped and carried by the velocity difference between the high-speed pulp flow and air flow, where air is suctioned at low pressure around the core area of the jet flow [
11]; a large portion of the bubbles generated in the jet suction mode have a small size [
12], such as nanobubbles, and were two orders of magnitude smaller than conventional sized bubbles [
10]. A Venturi type bubble generator can produce a large number of micro bubbles [
13] and the Jameson cell has a low energy consumption [
14]. It has been found that a rotational flow in the cyclonic-static micro-bubble flotation column is a benefit for the particles colliding with and attaching to the bubbles, and the probability of particle attachment substantially increased [
15]. A non-uniform filling method was proposed to optimize the flow field. Filling with a non-uniform sieve in the cyclonic-static micro-bubble flotation column was proved to be more effective on bubble size distribution equalization [
16]. In addition, it was found that rotational flow improves the effect of mineralization and increases the recovery of coarse coal particles in the cyclonic-static micro-bubble flotation column [
17]. Moreover, a nozzle equipped with a long throat and arranged in a submerged manner was studied [
18]. It was observed that the suction capacity of the nozzle increased with the nozzle distance and cross-section ratio. Furthermore, it resulted in a high jet kinetic energy dissipation rate. However, when exposed to air, either the suction capacity decreased significantly, or the nozzle failed to work properly.
As we all know, the processes of collision, detachment, and attachment between the bubbles and the coal particles complete the final coal slime flotation process [
19]. The bubble is the main carrier of the flotation process. The bubble size distribution has a significant influence on the selectivity of the bubble and the ability to carry the target mineral [
20]. Bubbles of different sizes have different abilities to separate minerals, which directly affects the efficiency of the flotation equipment [
21]. Therefore, it is extremely important to measure the BSD of the flotation device. There are many factors that affect the BSD, such as various frothers, frother concentration, impeller speed, aeration quantity, and solution pH, which further affect the generation of mineralized bubbles [
22,
23]. Compared to water alone, the addition of frother produces a narrower distribution and finer bubble size [
24]. Various frother types have different critical coalescence concentrations (CCCs). The impeller speed-bubble size tests show that
D32 is unaffected by increased impeller tip speed across the range of 4.6 to 9.2 m/s (representing the industrial operating range), although
D32 starts to increase below 4.6 m/s. The results suggest that the bubble size and bubble size distribution (BSD) do not change with increasing impeller speed in the quiescent zone of the flotation [
25]. The bubble size increases with the increase of gas rate, and, conversely, decreases with the increase in the impeller tip speed in the Denver mechanical flotation cell [
26]. As such, it is extremely important to measure the BSD and discuss its distribution regularity to evaluate the gas dispersion capacity of the flotation equipment [
27]. It is noteworthy that bubble coalescence is completely prevented as the frother concentration reaches the CCC [
28], and the bubble Sauter mean diameter remains almost constant [
24,
29].
These studies have only focused on various aspects of a single flotation machine; however, they failed to combine the characteristics of two or more flotation machines and conduct performance tests. Therefore, in this study, we developed a new jet-stirring flotation device that combines the advantageous characteristics of the three most common flotation machines. These advantages are that the Jameson cell made full use of jet flow to eject a large amount of air and produce smaller size bubbles; the stirring effect of a mechanical flotation cell made the slurry circulation effect better and the flotation was more sufficient; and the rotational flow of cyclonic-static micro-bubble flotation column improved the effect of mineralization and increased the mineral recovery rate. Further, we investigated the suction performance of its double cosine self-aspirated nozzle under various feeding pressures. The effects of the feeding pressure, frother concentration, suction capacity, and sampling location height on the bubble size in the flotation cell were also investigated via a bubble size measurement system.
5. Conclusions
In this study, a new jet-stirring coupling flotation device was designed. It was found that due to the arrangement of double cosine self-aspirated nozzles in a non-submerged manner, a large amount of air was sucked into the flotation cell, which met the requirements of flotation in terms of the suction capacity. D32 decreased with an increasing MIBC concentration until the concentration reached the CCC, above which the bubble size stabilized at approximately 0.31 mm, which was smaller than the bubble size produced by the conventional flotation machine. Higher MIBC concentrations led to the formation of a large number of small-sized bubbles. Moreover, D32 decreased with the increasing feeding pressure; conversely, it increased with suction capacity and sampling location height, independent of the frother concentration. As stated above, bubbles suitable for froth flotation were generated by the jet-stirring coupling flotation device, verifying the efficacy of the overall structural design of the device.
In this study, all the bubble tests of the jet-stirring coupling flotation device were carried out in the presence of the frother. It is well known that the agent used in the froth flotation also includes a collector. However, the BSD tests were not studied under the interaction of the two agents. Therefore, the shape characteristic test of the bubble will be carried out under the action of the collector and frother in future work.