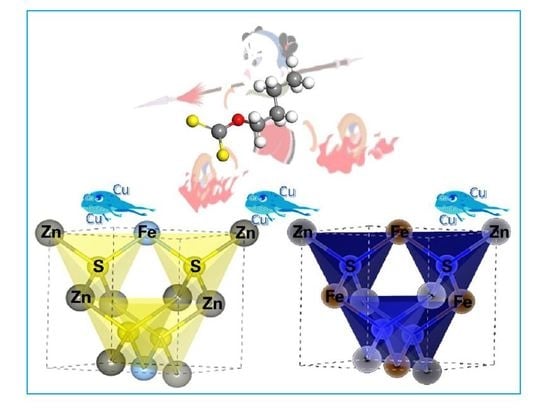
Mechanism Study of Xanthate Adsorption on Sphalerite/Marmatite Surfaces by ToF-SIMS Analysis and Flotation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Adsorption Capacity Measurements
2.3. Single Mineral Flotation
2.4. ToF-SIMS Analysis
3. Results
3.1. Effects of Fe Concentration on Xanthate Adsorption and Flotation Response
3.2. ToF-SIMS Spectra Analysis
3.3. ToF-SIMS Imaging Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fontboté, L.; Kouzmanov, K.; Chiaradia, M.; Pokrovski, G.S. Sulfide minerals in hydrothermal deposits. Elements 2017, 13, 97–103. [Google Scholar] [CrossRef]
- Deng, J.-S.; Mao, Y.-B.; Wen, S.-M.; Liu, J.; Xian, Y.-J.; Feng, Q.-C. New influence factor inducing difficulty in selective flotation separation of Cu-Zn mixed sulfide minerals. Int. J. Miner. Metall. Mater. 2015, 2, 111–115. [Google Scholar] [CrossRef]
- Thella, J.; Farrokhpay, S.; Nguyen, A.V. The influence of water quality on sulfide mineral flotation-a review. In Proceedings of the Asia Pacific Confederation of Chemical Engineering Congress 2015: APCChE 2015, Incorporating CHEMECA 2015, Melbourne, Australia, 27 September–1 October 2015; Engineers Australia: Barton, Australia, 2015; pp. 2405–2408. [Google Scholar]
- Nemchinova, L.; Zimin, A. Thermomechanical aspect of selective flotation of sphalerite and pyrite. Eurasian Min. 2015, 1, 19. [Google Scholar]
- Deng, J.; Lei, Y.; Wen, S.; Chen, Z. Modeling interactions between ethyl xanthate and Cu/Fe ions using DFT/B3LYP approach. Int. J. Miner. Process. 2015, 140, 43–49. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Wang, Y.; Deng, J.; Chen, X. Transition state search study on the migration of Cu absorbed on the S sites of sphalerite (110) surface. Int. J. Miner. Process. 2016, 147, 28–30. [Google Scholar] [CrossRef]
- Sarvaramini, A.; Larachi, F.; Hart, B. Collector attachment to lead-activated sphalerite–Experiments and DFT study on pH and solvent effects. Appl. Surf. Sci. 2016, 367, 459–472. [Google Scholar] [CrossRef]
- Deng, J.; Wen, S.; Xian, Y.; Liu, J.; Bai, S. New discovery of unavoidable ions source in chalcopyrite flotation pulp: Fluid inclusions. Miner. Eng. 2013, 42, 22–28. [Google Scholar] [CrossRef]
- Deng, J.; Wen, S.; Liu, J.; Xian, Y.; Wu, D.; Bai, S. New source of unavoidable ions in bornite flotation aqueous solution: Fluid inclusions. Ind. Eng. Chem. Res. 2013, 52, 4895–4901. [Google Scholar] [CrossRef]
- Deng, J.-S.; Wen, S.-M.; Wu, D.-D.; Liu, J.; Zhang, X.-L.; Shen, H.-Y. Existence and release of fluid inclusions in bornite and its associated quartz and calcite. Int. J. Miner. Metall. Mater. 2013, 20, 815–822. [Google Scholar] [CrossRef]
- Mohseni, M.; Abdollahi, M.; Poursalehi, R.; Khalesi, M.R. An insight into effect of surface functional groups on reactivity of Sphalerite (110) surface with Xanthate collector: A DFT study. J. Min. Environ. 2017, 9, 431–439. [Google Scholar]
- Long, X.; Chen, Y.; Chen, J.; Xu, Z.; Liu, Q.; Du, Z. The effect of water molecules on the thiol collector interaction on the galena (PbS) and sphalerite (ZnS) surfaces: A DFT study. Appl. Surf. Sci. 2016, 389, 103–111. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Luo, D.; Chen, L.; Deng, J. Comparative study on the copper activation and xanthate adsorption on sphalerite and marmatite surfaces. Appl. Surf. Sci. 2018, 439, 263–271. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Pattrick, R.A.; Mosselmans, J.F.W.; Charnock, J.M. An X-ray absorption study of doped sphalerites. Eur. J. Mineral. 1998, 10, 239–250. [Google Scholar] [CrossRef]
- Benedetto, F.D.; Andreozzi, G.; Bernadini, G.; Borgheresi, M.; Caneschi, A.; Cipciani, C.; Gatteschi, D.; Romanelli, M. Short range order of Fe2+ impurities, isolated and in pairs. ZnS and CdS studies by the Mossbauer effect. Phys. Chem. Miner. 2005, 32, 339–348. [Google Scholar] [CrossRef]
- Wright, K.; Gale, J.D. A first principles study of the distribution of iron in sphalerite. Geochim. Cosmochim. Acta 2010, 74, 3514–3520. [Google Scholar] [CrossRef]
- Quast, K.; Hobart, G. Marmatite depression in galena flotation. Miner. Eng. 2006, 19, 860–869. [Google Scholar] [CrossRef]
- Harmer, S.L.; Mierczynska-Vasilev, A.; Beattie, D.A.; Shapter, J.G. The effect of bulk iron concentration and heterogeneities on the copper activation of sphalerite. Miner. Eng. 2008, 21, 1005–1012. [Google Scholar] [CrossRef]
- Lepetit, P.; Bente, K.; Doering, T.; Luckhaus, S. Crystal chemistry of Fe-containing sphalerites. Phys. Chem. Miner. 2003, 30, 185–191. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Guo, J. A DFT study on the effect of lattice impurities on the electronic structures and floatability of sphalerite. Miner. Eng. 2010, 23, 1120–1130. [Google Scholar] [CrossRef]
- Szczypa, J.; Solecki, J.; Komosa, A. Effect of surface oxidation and iron contents on xanthate ions adsorption of synthetic sphalerites. Int. J. Miner. Process. 1980, 7, 151–157. [Google Scholar] [CrossRef]
- Boulton, A.B. Improving Sulphide Mineral Flotation Selectivity Against Iron Sulphide Gangue. Ph.D. Thesis, University of South Australia, Adelaide, Australia, 2002. [Google Scholar]
- Gigowski, B.; Vogg, A.; Wierer, K.; Dobias, B. Effect of Fe-lattice ions on adsorption, electrokinetic, calorimetric and flotation properties of sphalerite. Int. J. Miner. Process. 1991, 33, 103–120. [Google Scholar] [CrossRef]
- Chandra, A.P.; Gerson, A.R. A review of the fundamental studies of the copper activation mechanisms for selective flotation of the sulfide minerals, sphalerite and pyrite. Adv. Colloid Interface Sci. 2009, 145, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.; Fornasiero, D.; Ralston, J. Effect of iron content in sphalerite on flotation. Miner. Eng. 2005, 18, 1120–1122. [Google Scholar] [CrossRef]
- Yu, R.; Qiu, G.; Hu, Y.; Qin, W. Adsorption mechanism of ethyl xanthate on marmatite. Metal Mine 2004, 29–31. (In Chinese) [Google Scholar]
- Zhang, Q.; Hu, Y.; Xu, J.; Chen, T. Study of the marmatite collectorless flotation. Nonferrous Met. Mieral Process. Sect. 2005, 19–21. (In Chinese) [Google Scholar]
- Zhang, Q.; Yue-Hua, H.U.; Guo-Hua, G.U.; Jing, X.U. Mechanism of Cu2+ ion activation flotation of marmatite in absence and presence of ethyl xanthate. Chin. J. Nonferrous Met. 2004, 14, 676–680. (In Chinese) [Google Scholar]
- Pattrick, R.A.D.; England, K.E.R.; Charnock, J.M.; Mosselmans, J.F.W. Copper activation of sphalerite and its reaction with xanthate in relation to flotation: An X-ray absorption spectroscopy (reflection extended X-ray absorption fine structure) investigation. Int. J. Miner. Process. 1999, 55, 247–265. [Google Scholar] [CrossRef]
- Khmeleva, T.N.; Chapelet, J.K. Depression mechanisms of sodium bisulphite in the xanthate-induced flotation of copper activated sphalerite. Int. J. Miner. Process. 2006, 79, 61–75. [Google Scholar] [CrossRef]
- Mikhlin, Y.; Karacharov, A.; Tomashevich, Y.; Shchukarev, A. Interaction of sphalerite with potassium n-butyl xanthate and copper sulfate solutions studied by XPS of fast-frozen samples and zeta-potential measurement. Vacuum 2016, 125, 98–105. [Google Scholar] [CrossRef]
- Popov, S.R.; Vučinić, D.R. Floatability and adsorption of ethyl xanthate on copper-activated sphalerite in weakly acidic medium. Colloids Surf. 1990, 47, 81–94. [Google Scholar] [CrossRef]
- Sodhi, R.N. Time-of-flight secondary ion mass spectrometry (TOF-SIMS):—Versatility in chemical and imaging surface analysis. Analyst 2004, 129, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, K.L.; Wark, I.W. Principles of Flotation; Australasian Institute of Mining and Metallurgy: Carlton, Victoria, Australia, 1995. [Google Scholar]
- Ejtemaei, M.; Nguyen, A.V. Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 100, 223–232. [Google Scholar] [CrossRef]
- Gerson, A.R.; Lange, A.G.; Prince, K.E.; Smart, R.S.C. The mechanism of copper activation of sphalerite. Appl. Surf. Sci. 1999, 137, 207–223. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Y.; Zeng, X.; Li, Y. First principle study of effect of Fe impurity on electronic structure and activation of sphalerite surface. Chin. J. Nonferrous Met. 2009, 19, 1517–1523. (In Chinese) [Google Scholar]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.; Deng, J.; Fan, G.; Xu, H.; Chen, W.; Li, S.; Huang, L. Mechanism Study of Xanthate Adsorption on Sphalerite/Marmatite Surfaces by ToF-SIMS Analysis and Flotation. Minerals 2019, 9, 205. https://doi.org/10.3390/min9040205
Lai H, Deng J, Fan G, Xu H, Chen W, Li S, Huang L. Mechanism Study of Xanthate Adsorption on Sphalerite/Marmatite Surfaces by ToF-SIMS Analysis and Flotation. Minerals. 2019; 9(4):205. https://doi.org/10.3390/min9040205
Chicago/Turabian StyleLai, Hao, Jiushuai Deng, Guixia Fan, Hongxiang Xu, Wenxiang Chen, Shimei Li, and Lingyun Huang. 2019. "Mechanism Study of Xanthate Adsorption on Sphalerite/Marmatite Surfaces by ToF-SIMS Analysis and Flotation" Minerals 9, no. 4: 205. https://doi.org/10.3390/min9040205