Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mineral Sorbent Sources
2.2. General Characterization of the Mineral Sorbents
2.3. Sorption Experiments
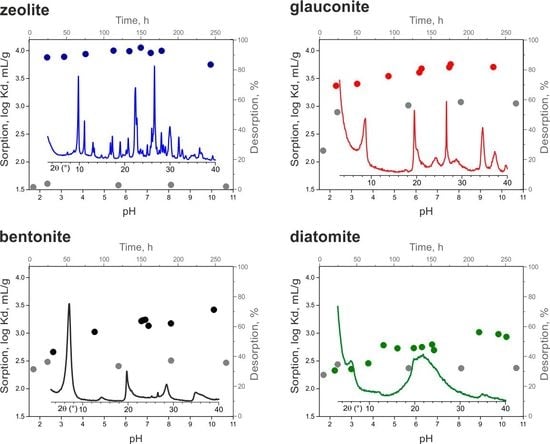
3. Results and Discussion
3.1. Mineral Sorbents Characterization
3.2. Characterization of Specific Surface Area and Porosity
3.3. Cation Exchange Capacity
3.4. Cesium Sorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bergaya, F.; Theng, B.K.G.; Lagaly, G. Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, p. 1246. [Google Scholar]
- Grauer, R. Bentonite as a backfill material in a high- level waste repository. MRS Bull. 1994, 19, 43–46. [Google Scholar] [CrossRef]
- Sellin, P.; Leupin, O.X. The Use of Clay as an Engineered Barrier in Radioactive-Waste Management—A Review. Clays Clay Miner. 2013, 61, 477–498. [Google Scholar] [CrossRef]
- Guggenheim, S.; Adams, J.M.; Bain, D.C.; Bergaya, F.; Brigatti, M.F.; Drits, V.A.; Formoso, M.L.L.; Galan, E.; Kogure, T.; Stanjek, H. Summary of recommendations of Nomenclature Committees relevant to clay mineralogy: Report of the Association Internationale Pour L’etude des Argiles (AIPEA) nomenclature committee for 2006. Clays Clay Miner. 2006, 54, 761–772. [Google Scholar] [CrossRef]
- Osmanlioglu, A.E. Natural diatomite process for removal of radioactivity from liquid waste. Appl. Radiat. Isot. 2007, 65, 17–20. [Google Scholar] [CrossRef]
- Taylor, P.; Mimura, H.; Kanno, T. Distribution and Fixation of Cesium and Strontium in Zeolite A and Chabazite. J. Nucl. Sci. Technol. 1985, 22, 284–291. [Google Scholar]
- El-Kamash, A.M. Evaluation of zeolite A for the sorptive removal of Cs+and Sr2+ ions from aqueous solutions using batch and fixed bed column operations. J. Hazard. Mater. 2008, 151, 432–445. [Google Scholar] [CrossRef]
- Borai, E.H.; Harjula, R.; Malinen, L.; Paajanen, A. Efficient removal of cesium from low-level radioactive liquid waste using natural and impregnated zeolite minerals. J. Hazard. Mater. 2009, 172, 416–422. [Google Scholar] [CrossRef]
- Nenadović, S.; Kljajević, L.; Marković, S.; Omerašević, M.; Jovanović, U.; Andrić, V.; Vukanac, I. Natural diatomite (Rudovci, Serbia) as adsorbent for removal Cs from radioactive waste liquids. Sci. Sinter. 2015, 47, 299–309. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C., Jr. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: Oxford, UK, 1999; p. 378. [Google Scholar]
- Post, J.E.; Bish, D.L. Rietveld refinement of crystal structures using powder X-ray diffraction data. Rev. Miner. Geochem. 1989, 20, 277–308. [Google Scholar]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Cryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Cokca, E.; Birand, A. Determination of Cation Exchange Capacity of Clayey Soils by the Methylene Blue Test. Geotech. Test. J. 1993, 16, 518–524. [Google Scholar]
- Alexander, E.B. Cation-exchange capacity of acid soils Using aluminum chloride and barium chloride-trietha nolamine. Soil Sci. Soc. Am. J. 1976, 40, 961–963. [Google Scholar] [CrossRef]
- Tucker, B.M. Laboratory Procedures for Cation Exchange Measurement on Soils. Available online: https://trid.trb.org/view/37268 (accessed on 10 October 2019).
- Semenkova, A.S.; Evsiunina, M.V.; Verma, P.K.; Mohapatra, P.K.; Petrov, V.G.; Seregina, I.F.; Bolshov, M.A.; Krupskaya, V.V.; Romanchuk, A.Y.; Kalmykov, S.N. Cs+ sorption onto Kutch clays: Influence of competing ions. Appl. Clay Sci. 2018, 166, 88–93. [Google Scholar] [CrossRef]
- Goldberg, S.; Criscenti, L.J.; Turner, D.R.; Davis, J.A.; Cantrell, K.J. Adsorption–Desorption Processes in Subsurface Reactive Transport Modeling. Vadose Zone J. 2007, 6, 407–435. [Google Scholar] [CrossRef]
- Payne, T.E.; Brendler, V.; Ochs, M.; Baeyens, B.; Brown, P.L.; Davis, J.A.; Ekberg, C.; Kulik, D.A.; Lutzenkirchen, J.; Missana, T.; et al. Guidelines for thermodynamic sorption modelling in the context of radioactive waste disposal. Environ. Model. Softw. 2013, 42, 143–156. [Google Scholar] [CrossRef]
- Geckeis, H.; Lützenkirchen, J.; Polly, R.; Rabung, T.; Schmidt, M. Mineral−Water Interface Reactions of Actinides. Chem. Rev. 2013, 113, 1016–1062. [Google Scholar] [CrossRef]
- Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms, 2nd ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 2002; p. 486.
- Farmer, V.C. The Infrared Spectra of Minerals; Monograph 4, Book Section 16; Mineralogical Society: London, UK, 1974; p. 539. [Google Scholar]
- Zviagina, B.B.; Drits, V.A.; Sakharov, B.A.; Ivanovskaya, T.A.; Dorzhieva, O.V.; McCarty, D.K. Crystal-chemical regularities and identification criteria in Fe-bearing K-dioctahedral micas 1M from X-ray diffraction and Infrared spectroscopy data. Clays Clay Miner. 2017, 65, 234–251. [Google Scholar] [CrossRef]
- Madejovà, J.; Komadel, P. Baseline studies of The Clay Minerals Society Source Clays: Infrared Methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Madejovà, J.; Komadel, P. Information available from infrared spectra of the fine fractions of bentonites. In The Application of Vibrational Spectroscopy to Clay Minerals and Layered Double Hydroxides; CMS Workshop Lectures; The Clay Mineral Society: Aurora, CO, USA, 2005; Volume 13, pp. 65–98. [Google Scholar]
- Smith, D.K. Opal, cristobalite, and tridymite: Noncrystallinity versus crystallinity, nomenclature of the silica minerals and bibliography. Powder Diffr. 1998, 13, 2–19. [Google Scholar] [CrossRef]
- Guatame-Garcia, A.; Buxton, M. The Use of Infrared Spectroscopy to Determine the Quality of Carbonate-Rich Diatomite Ores. Minerals 2018, 8, 120. [Google Scholar] [CrossRef]
- Van Olphen, H. An Introduction to Clay Colloid Chemistry; Wiley-Interscience: New York, NY, USA, 1963; p. 301. [Google Scholar]
- Lagaly, G.; Ziesmer, S. Colloid chemistry of clay minerals: The coagulation of montmorillonite dispersions. Adv. Colloid Interface Sci. 2003, 100–102, 105–128. [Google Scholar] [CrossRef]
- IUPAC. Manual of Symbols and Terminology. Pure Appl. Chem. 1972, 31, 577. [Google Scholar]
- Rouquerolt, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.H.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of 830 porous solids. Pure Appl. Chem. 1997, 66, 1739–1758. [Google Scholar] [CrossRef]
- Karnauhov, A.P. Adsorption. In Texture of Dispersive and Porous Materials; Nauka: Novosibirsk, Russia, 1999; p. 470. (In Russian) [Google Scholar]
- Osipov, V.I.; Sokolov, V.N. Clays and their properties. In Composition, Structure and Formation of Properties; GEOS: Moscow, Russia, 2013; p. 576. [Google Scholar]
- Haggerty, G.M.; Bowman, R.S. Sorption of chromate and other inorganic anions by organo-zeolite. Environ. Sci. Technol. 1994, 28, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Rikhtegar, N.; Panahi, H.; Atabi, F.; Karimi, S.B. Porosity, characterization and structural properties of natural zeolite—clinoptilolite—As a sorbent. Environ. Prot. Eng. 2013, 39, 139. [Google Scholar]
- Ivanovskaya, T.A.; Zvyagina, B.B.; Sakharov, B.A.; Zaitseva, T.S. Crystal-chemical peculiarities of globular layer silicates of the glauconite-illite composition (Upper Proterozoic, Northern Siberia). Lithol. Miner. Resour. 2012, 47, 491–512. [Google Scholar] [CrossRef]
- Zaunbrecher, K.L.; Cygan, R.; Elliott, W.C. Molecular Models of Cesium and Rubidium Adsorption on Weathered Micaceous Minerals. J. Phys. Chem. A 2015, 119, 5691–5700. [Google Scholar] [CrossRef]
- Dohrmann, R.; Genske, D.; Karnland, O.; Kaufhold, S. Interlaboratory CEC and exchangeable cation study of bentonite buffer materials: I. Cu(II)-triethylenetetramine method. Clays Clay Miner. 2012, 60, 162–175. [Google Scholar] [CrossRef]
- Lieser, Κ.H.; Steinkopff, T. Chemistry of Radioactive Cesium in the Hydrosphere and in the Geosphere. Radiochim. Acta 1989, 46, 39–47. [Google Scholar] [CrossRef]
- Wissocq, A.; Beaucaire, C.; Latrille, C. Application of the multi-site ion exchanger model to the sorption of Sr and Cs on natural clayey sandstone. Appl. Geochem. 2018, 1, 11. [Google Scholar] [CrossRef]
- Comans, N.J.; Haller, R.; De Preter, M.P. Sorption of cesium on illite: Non-equilibrium behaviour and reversibility. Geochim. Cosmochim. Acta 1991, 55, 433–440. [Google Scholar] [CrossRef]
- Krupskaya, V.; Zakusin, S.; Tyupina, E.; Dorzhieva, O.; Zhukhlistov, A.; Belousov, P.; Timofeeva, M. Experimental Study of Montmorillonite Structure and Transformation of Its Properties under Treatment with Inorganic Acid Solutions. Minerals 2017, 7, 49. [Google Scholar] [CrossRef]
- Krupskaya, V.V.; Zakusin, S.V.; Tyupina, E.A.; Dorzhieva, O.V. Transformation of Structure and Adsorption Properties of Montmorillonite under Thermochemical Treatment. Geochem. Int. 2019, 57, 314–330. [Google Scholar] [CrossRef]
- Krupskaya, V.; Novikova, L.; Tyupina, E.; Belousov, P.; Dorzhieva, O.; Zakusin, S.; Kim, K.; Roessner, F.; Badetti, E.; Brunelli, A.; et al. The influence of acid modification on the structure of montmorillonites and surface properties of bentonites. Appl. Clay Sci. 2019, 172, 1–10. [Google Scholar] [CrossRef]
- Puigdomenech, I. Make Equilibrium Diagrams Using Sophisticated Algorithms (MEDUSA). In Inorganic Chemistry; Royal Institute of Technology: Stockholm, Sweden, 2004. [Google Scholar]
- Song, K.C.; Lee, H.K.; Moon, H.; Lee, K.J. Simultaneous removal of the radiotoxic nuclides Cs-137 and I-129 from aqueous solution. Sep. Purif. Technol. 1997, 12, 215–227. [Google Scholar] [CrossRef]
- Englehardt, D. Original Contribution Pozzolanic Filtration/Solidification of Radionuclides in Nuclear Reactor Cooling Water. Waste Manag. 1996, 15, 585–592. [Google Scholar] [CrossRef]
- Bradbury, M.H.; Baeyens, B.A. Generalised sorption model for the concentration dependent uptake of caesium by argillaceous rocks. J. Contam. Hydrol. 2000, 42, 141–163. [Google Scholar] [CrossRef]
- Durrant, C.B.; Begg, J.D.; Kersting, A.B.; Zavarin, M. Cesium sorption reversibility and kinetics on illite, montmorillonite, and kaolinite. Sci. Total Environ. 2018, 610–611, 511–520. [Google Scholar] [CrossRef]
- Missana, T.; García-Gutiérrez, M.; Benedicto, A.; Ayora, C.; De-Pourcq, K. Modelling of Cs sorption in natural mixed-clays and the effects of ion competition. Appl. Geochem. 2014, 49, 95–102. [Google Scholar] [CrossRef]
- Poinssot, C.; Baeyens, B.; Bradbury, M.H. Experimental and modeling studies of caesium sorption on illite. Clays Clay Miner. 1999, 63, 3217–3227. [Google Scholar]
- Tachi, Y.; Yotsuji, K.; Seida, Y.; Yui, M. Diffusion and sorption of Cs+, I−, and HTO in samples of the argillaceous Wakkanai formation from the Horonobe URL, Japan: Clay-based modeling approach. Geochem. Cosmochim. Acta 2011, 75, 6742–6759. [Google Scholar] [CrossRef]
- Bostick, B.; Vairavamurthy, M.; Karthikeyan, K.; Chorover, J. Cesium Adsorption on Clay Minerals: An EXAFS Spectroscopic Investigation. Environ. Sci. Technol. 2002, 36, 2670–2676. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Tanaka, M.; Kurihara, Y.; Takahashi, Y. Local structure of strontium adsorbed on 2:1 clay minerals and itscomparison with cesium by XAFS in terms of migration of theirradioisotopes in the environment. J. Radioanal. Nucl. Chem. 2018, 17, 545–551. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Loganathan, N.; Wakou, B.F.N.; Chen, Z. Interaction of ions with hydrated clay surfaces: Computational molecular modeling for nuclear waste disposal applications. Proc. Earth Planet. Sci. 2017, 17, 566–569. [Google Scholar] [CrossRef]
- Loganathan, N.; Kalinichev, A.G. Quantifying the Mechanisms of Site-Specific Ion Exchange at an Inhomogeneously Charged Surface: Case of Cs+/K+ on Hydrated Muscovite Mica. J. Phys. Chem. 2017, 121, 7829–7836. [Google Scholar] [CrossRef]
- Iijima, K.; Tomura, T.; Shoji, Y. Reversibility and modeling of adsorption behavior of cesium ions on colloidal montmorillonite particles. Appl. Clay Sci. 2010, 49, 262–268. [Google Scholar] [CrossRef]
- Missana, T.; García-Gutiérrez, M.; Alonso, Ú. Kinetics and irreversibility of cesium and uranium sorption onto bentonite colloids in a deep granitic environment. Appl. Clay Sci. 2004, 26, 137–150. [Google Scholar] [CrossRef]
- Fukushi, K.; Sakai, H.; Itono, T.; Tamura, A.; Arai, S. Desorption of intrinsic cesium from smectite: Inhibitive effects of clay particle organization on cesium desorption. Environ. Sci. Technol. 2014, 48, 10743–10749. [Google Scholar] [CrossRef]
- Fukushi, K.; Fukiage, T. Prediction of Intrinsic Cesium Desorption from Na-Smectite in Mixed Cation Solutions. Environ. Sci. Technol. 2015, 49, 17. [Google Scholar] [CrossRef]
- Nakao, A.; Thiry, Y.; Funakawa, S.; Kosaki, T. Characterization of the frayed edge site of micaceous minerals in soil clays influenced by different pedogenetic conditions in Japan and northern Thailand. Soil Sci. Plant Nutr. 2008, 54, 479–489. [Google Scholar] [CrossRef] [Green Version]





| Sample Name | LOI | Na2O | MgO | Al2O3 | SiO2 | K2O | CaO | TiO2 | MnO | Fe2O3 | P2O5 | SO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glauconite | 9.94 | 0.07 | 3.75 | 7.61 | 50.52 | 6.94 | 0.59 | 0.12 | 0.016 | 19.72 | 0.21 | <0.02 |
| Bentonite | 20.40 | 11.48 | 2.35 | 16.09 | 44.63 | 0.32 | 0.17 | 0.55 | 0.015 | 2.93 | 0.07 | <0.02 |
| Zeolite | 8.56 | 1.48 | 0.65 | 13.14 | 68.62 | 3.35 | 2.38 | 0.16 | 0.041 | 1.11 | 0.02 | <0.02 |
| Diatomite | 10.87 | 0.08 | 0.29 | 4.31 | 81.9 | 0.1 | 0.38 | 0.13 | 0.02 | 1.79 | 0.04 | <0.02 |
| Mineral | Glauconite | Bentonite | Zeolite | Diatomite |
|---|---|---|---|---|
| Glauconite | 99.6 | |||
| Opal | 80.8 | |||
| Clinoptilolite | 71.8 | |||
| Smectite | 96.8 | 1.4 | 9.9 | |
| Illite | 8.4 | |||
| Kaolinite * | 9.0 | |||
| Quartz | 0.4 | 2.4 | 15.0 | 0.3 |
| Anatase | 0.8 | |||
| Albite | 3.4 | |||
| Total content | 100 | 100 | 100 | 100 |
| Sample | Specific Surface Area SBET, m2/g | Pores Volum cm3/g, nm | Average Diameter, nm | Microporosity (Volume) (Т-Method Halsey), cm3/g | Specific Surface Area (Т-method Halsey), SBET, m2/g | ||
|---|---|---|---|---|---|---|---|
| Micropores | Meso-Macropores | Total | |||||
| Glauconite | 48 | 0.061 | 5.28 | 0.013 | 23 | 25 | 48 |
| Bentonite | 45 | 0.078 | 5.00 | 0.016 | 23 | 22 | 45 |
| Zeolite | 14 | 0.053 | 4.89 | - | - | - | 14 |
| Diatomite | 42 | 0.135 | 4.89 | - | - | - | 42 |
| Adsorbate/Index Cation | |||
|---|---|---|---|
| Sample | Ba2+ | MB (C16H18N3S+) | NH4+ |
| Glauconite | 12.2 | 16.6 | |
| Bentonite | 93.5 | ||
| Zeolite | 17.6 | <3 | 161.0 |
| Diatomite | 11.3 | 9.8 | |
| Sorbent | log Kd, mL/g |
|---|---|
| Glauconite | 3.6 |
| Bentonite | 2.8 |
| Zeolite | 4.0 |
| Diatomite | 2.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belousov, P.; Semenkova, A.; Egorova, T.; Romanchuk, A.; Zakusin, S.; Dorzhieva, O.; Tyupina, E.; Izosimova, Y.; Tolpeshta, I.; Chernov, M.; et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals 2019, 9, 625. https://doi.org/10.3390/min9100625
Belousov P, Semenkova A, Egorova T, Romanchuk A, Zakusin S, Dorzhieva O, Tyupina E, Izosimova Y, Tolpeshta I, Chernov M, et al. Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite. Minerals. 2019; 9(10):625. https://doi.org/10.3390/min9100625
Chicago/Turabian StyleBelousov, Petr, Anna Semenkova, Tolganay Egorova, Anna Romanchuk, Sergey Zakusin, Olga Dorzhieva, Ekaterina Tyupina, Yulia Izosimova, Inna Tolpeshta, Michail Chernov, and et al. 2019. "Cesium Sorption and Desorption on Glauconite, Bentonite, Zeolite, and Diatomite" Minerals 9, no. 10: 625. https://doi.org/10.3390/min9100625