Stability of Naturally Relevant Ternary Phases in the Cu–Sn–S System in Contact with an Aqueous Solution
Abstract
:1. Introduction
2. State-of-the-Art in the Cu–Sn–S Compositional Field
3. Modelling of the Equilibria in Aqueous Solutions
Solid Phases Considered in the Calculations
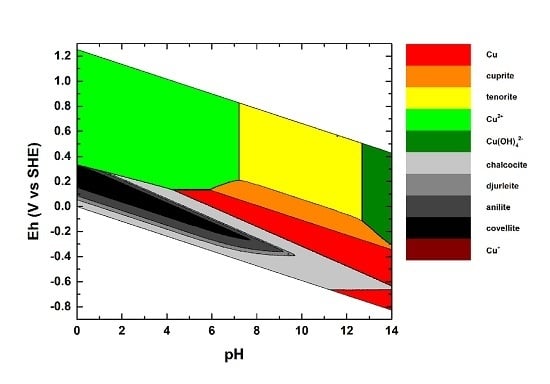
4. Test Systems (Cu–O–H, Sn–O–H, Cu–S–O–H, Sn–S–O–H)
5. The Study of the Cu–Sn–S–O–H System
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zawadzki, P.; Baranowski, L.L.; Peng, H.; Toberer, E.S.; Ginley, D.S.; Tumas, W.; Zakutayev, A.; Lany, S. Evaluation of photovoltaic materials within the Cu–Sn–S family. Appl. Phys. Lett. 2013, 103, 253902. [Google Scholar] [CrossRef]
- Tipcompor, N.; Thongtem, S.; Thongtem, T. Effect of microwave radiation on the morphology of tetragonal Cu3SnS4 synthesized by refluxing method. Superlattices Microstruct. 2015, 85, 488–496. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, L.; Yin, Y.; Qian, X.; Zhou, G.; Gu, X.; Liu, W.; Wu, X.; Zhang, F. Strong quantum confinement effect in Cu4SnS4 quantum dots synthesized via an improved hydrothermal approach. J. Alloy. Compd. 2016, 672, 204–211. [Google Scholar] [CrossRef]
- Tan, Q.; Sun, W.; Li, Z.; Li, J.-F. Enhanced thermoelectric properties of earth-abundant Cu2SnS3 via in doping effect. J. Alloy. Compd. 2016, 672, 558–563. [Google Scholar] [CrossRef]
- Baranowski, L.L. Combinatorial Development of Cu2SnS3 as an Earth Abundant Photovoltaic Absorber. Ph.D. Thesis, Colorado School of Mines, Golden, CO, USA, 2015; p. 143. [Google Scholar]
- Lokhande, A.C.; Gurav, K.V.; Jo, E.; Lokhande, C.D.; Hyeok Kim, J. Chemical synthesis of Cu2SnS3 (CTS) nanoparticles: A status review. J. Alloy. Compd. 2016, 656, 295–310. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Bencistà, I.; D’Acapito, F.; Frizzera, S.; Caneschi, A.; Innocenti, M.; Lavacchi, A.; Montegrossi, G.; Oberhauser, W.; Romanelli, M.; et al. Geomaterials related to photovoltaics: A nanostructured Fe-bearing kuramite, Cu3SnS4. Phys. Chem. Miner. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Moh, G.H. Tin-containing mineral systems. Part I: The Sn–Fe–S–O system and mineral assemblages in ores. Chemie Der Erde 1974, 33, 243–273. [Google Scholar]
- Wang, N. The three ternary phases in the system Cu–Sn–S. N. Jb. Miner. Mh. 1974, 9, 424–431. [Google Scholar]
- Khanafer, W.; Rivet, J.; Flahaut, J. Etude du ternaire Cu–Sn–S. Diagrammes d’equilibre des systémes Cu2S–SnS, Cu2S–Sn2S3 et Cu2S–SnS2. Etude cristallographique des composés Cu4SnS4, Cu2SnS3, Cu2Sn4S9, et Cu4Sn3S8. Bull. Soc. Chem. France 1974, 12, 267–276. (In French) [Google Scholar]
- Moh, G.H. Tin-containing mineral systems. Part II: Phase relations and mineral assemblage in the Cu–Fe–Zn–S system. Chemie Der Erde 1975, 34, 1–61. [Google Scholar]
- Wang, N. Idaite and the synthetic phases Cu4.33Ge0.67S5 and Cu9.67Sn2.33S13. N. Jb. Miner. Mh. 1976, 1976, 241–247. [Google Scholar]
- Sobott, R.J.G.; Teh, G.H. Investigations along the Cu2S–SnS2 join in the Cu–Sn–S system. N. Jb. Miner. Abh. 1977, 131, 23–26. [Google Scholar]
- Wang, N. Structural variations of non-stoichiometric Cu2SnS3. N. Jb. Miner. Abh. 1977, 131, 26–27. [Google Scholar]
- Jaulmes, S.; Rivet, J.; Laruelle, P. Cuivre–Etain–Soufre Cu4SnS4. Acta Cryst. B Struct. Crystallogr. Cryst. Chem. 1977, 33, 540–542. [Google Scholar] [CrossRef]
- Wang, N. Covellite related phases within the Cu–Fe–Sn–S system. N. Jb. Miner. Mh. 1981, 1981, 337–343. [Google Scholar]
- Wang, N. Sulfidization experiments performed at low temperatures. N. Jb. Miner. Abh. 1982, 144, 319–324. [Google Scholar]
- Jaulmes, J.; Rivet, J.; Jumas, M. Structure Cristalline du Sulfure et D’Etain CuSn3,75S8. Acta Cryst. B Struct. Crystallogr. Cryst. Chem. 1982, 38, 51–54. (In French) [Google Scholar] [CrossRef]
- Wu, D.; Knowles, C.R.; Chang, L.L.Y. Copper-tin sulphides in the system Cu–Sn–S. Miner. Mag. 1986, 50, 323–325. [Google Scholar] [CrossRef]
- Osadchii, E.G. Solid solutions kesterite–Mn–stannite and sphalerite–alabandite in the pseudoternary system Cu2SnS3–ZnS–MnS at 820 °C and 700 °C. N. Jb. Miner. Mh. 1996, 6, 201–211. [Google Scholar]
- Chen, X.; Wada, H.; Sato, A.; Mieno, M. Synthesis, Electrical Conductivity, and Crystal Structure of Cu4Sn7S16 and Structure Refinement of Cu2SnS3. J. Solid State Chem. 1998, 139, 144–151. [Google Scholar] [CrossRef]
- Chen, X.; Wada, H.; Sato, A. Preparation, crystal structure and electrical properties of Cu4SnS6. Mater. Res. Bull. 1999, 34, 239–247. [Google Scholar] [CrossRef]
- Olekseyuk, I.D.; Dudchak, I.V.; Piskach, L.V. Phase equilibria in the Cu2S–ZnS–SnS2 system. J. Alloy. Compd. 2004, 368, 135–143. [Google Scholar] [CrossRef]
- Fiechter, S.; Martinez, M.; Schmidt, G.; Henrion, W.; Tomm, Y. Phase relations and optical properties of semiconducting ternary sulfides in the system Cu–Sn–S. J. Phys. Chem. Solids 2003, 64, 1859–1862. [Google Scholar] [CrossRef]
- Jemetio, J.P.F.; Zhou, P.; Kleinke, H. Crystal structure, electronic structure and thermoelectric properties of Cu4Sn7S16. J. Alloy. Compd. 2006, 417, 55–59. [Google Scholar] [CrossRef]
- Kovalenker, V.A.; Malov, V.S.; Evstigneeva, T.L.; Vyal’sov, L.N. Mohite, Cu2SnS3, a new sulfide of tin and copper. Zapiski. Vses. Mineralog. Obshch. 1982, 111, 110–114. (In Russian) [Google Scholar] [CrossRef]
- Pascua, M.I.; Murciego, A.; Pellittero, E. Sn–Ge–Gd–Cu–Fe-Bearing Sulfides and Sulfosalts from the Barouilla Deposit, Salamanca, Spain. Can. Miner. 1997, 35, 39–52. [Google Scholar]
- Kovalenker, V.A.; Nekrasov, I.Y.; Malov, V.S. Copper and iron sulfostannate minerals in gold-silver deposits. Int. Geol. Rev. 1986, 28, 1443–1459. [Google Scholar] [CrossRef]
- Kovalenker, V.A. Kuramite, Cu3SnS4, a new mineral of the stannite group. Int. Geol. Rev. 1981, 23, 365–370. [Google Scholar] [CrossRef]
- Franchini, M.; Impiccini, A.; O’Leary, S.; Ríos, F.J.; Schalamuk, A.I. Distribución de las alteraciones y mineralizaciones en la sección central del yacimiento Agua Rica (27°22′ S–66°16′ O), Catamarca. Rev. Asoc. Geol. Argent. 2009, 64, 391–408. (In Spanish) [Google Scholar]
- Franchini, M.; Impiccini, A.; Lentz, D.; Ríos, F.J.; O’Leary, S.; Pons, J.; Schalamuk, A.I. Porphyry to epithermal transition in the Agua Rica polymetallic deposit, Catamarca, Argentina: An integrated petrologic analysis of ore and alteration parageneses. Ore Geol. Rev. 2011, 41, 49–74. [Google Scholar] [CrossRef]
- Huang, H.H. The Eh-pH Diagram and Its Advances. Metals 2016, 6, 23. [Google Scholar] [CrossRef]
- Parkhurst, D.L.; Appelo, C.A.J. User’s Guide to PHREEQC (Version 2)—A Computer program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations; Water-Resources Investigations Report 99-4259; U.S. Geological Survey, Earth Science Information Center, Open-File Reports Section: Reston, VA, USA, 1999.
- Kinniburgh, D.; Cooper, D. PhreePlot: Creating Graphical Output with PHREEQC. Available online: http://nora.nerc.ac.uk/19744/1/PhreePlot.pdf (accessed on 8 June 2016).
- Helgeson, H.C.; Delaney, J.M.; Nesbitt, H.W.; Bird, D.K. Summary and critique of the thermodynamic properties of rock forming minerals. Am. J. Sci. 1978, 278, 1–229. [Google Scholar]
- Johnson, J.; Oelkers, E.; Helgeson, H. SUPCRT92: A software package for calculating the standard molal thermodynamic properties of minerals, gases, aqueous species and reactions from 1 to 5000 bar and 0 to 1000 °C. Comp. Geosci. 1992, 18, 899–947. [Google Scholar] [CrossRef]
- Ball, J.W.; Nordstrom, D.K. User’s Manual for WATEQ4F, with Revised Thermodynamic Data Base and Text Cases for Calculating Speciation of Major, Trace, and Redox Elements in Natural Waters; USGS Numbered Series Open-File Report 91-183; U.S. Geological Survey: Reston, VA, USA, 1991; p. 193.
- Delany, J.M.; Lundeen, S.R. The LLNL Thermochemical Database: Revised Data and File Format for the EQ3/6 Package; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1991. [Google Scholar]
- Stull, D.R.; Prophet, H. JANAF Thermochemical Tables, 2nd ed.; Office of Standard Reference Data, National Bureau of Standards: Washington, DC, USA, 1971; Volume 37, p. 1141. [Google Scholar]
- Robie, R.A.; Waldbaum, D.R. Thermodynamic Properties of Minerals and Related Substances at 298.15 K (25.0 °C) and One Atmosphere (1.013 Bars) Pressure and at Higher Temperatures; U.S. Government Printing Office: Washington, DC, USA, 1968.
- Moody, G.J.; Thomas, J.D.R. Lattice energy and chemical prediction: Use of the Kapustinskii Equations and the Born-Haber cycle. J. Chem. Educ. 1965, 42, 204–210. [Google Scholar] [CrossRef]
- Donald, H.; Jenkins, B. Thermodynamics of the Relationship between Lattice Energy and Lattice Enthalpy. J. Chem. Educ. 2005, 82, 950–952. [Google Scholar]
- Jackson, A.J.; Walsh, A. Ab initio thermodynamic model of Cu2ZnSnS4. J. Mater. Chem. A 2014, 2, 7829–7836. [Google Scholar] [CrossRef] [Green Version]
- Onoda, M.; Chen, X.; Sato, A.; Wada, H. Crystal structure and twinning of monoclinic Cu2SnS3. Mater. Res. Bull. 2000, 35, 1563–1570. [Google Scholar] [CrossRef]
- Vieillard, P.; Tardy, Y. Prediction of enthalpy of formation based on refined crystal structures of multisite compounds. 2. Application to minerals belonging to the system Li2O-Na2O-K2O-BeO-MgO-CaO-MnO-FeO-Fe2O3-Al2O3-SiO2-H2O. Results and discussion. Geochim. Cosmochim. Acta 1994, 58, 4064–4107. [Google Scholar] [CrossRef]
- Vieillard, P. Prediction of enthalpy of formation based on refined cyrstal structures of multisite compounds. 1. Theories and examples. Geochim. Cosmochim. Acta 1994, 58, 4049–4063. [Google Scholar] [CrossRef]
- Burton, L.A.; Walsh, A. Phase Stability of the Earth-Abundant Tin Sulfides SnS, SnS2, and Sn2S3. J. Phys. Chem. C 2012, 116, 24262–24267. [Google Scholar] [CrossRef] [Green Version]
- Baranowski, L.; Zawadzki, P.; Christensen, S.; Nordlund, D.; Lany, S.; Tamboli, A.C.; Gedvilas, L.; Ginley, D.S.; Tumas, W.; Toberer, E.S.; et al. Control of Doping in Cu2SnS3 through Defects and Alloying. Chem. Mater. 2014, 26, 4951–4959. [Google Scholar] [CrossRef]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer Science & Business Media: Berlin, Heidelberg, Germany, 1988; p. 176. [Google Scholar]
- Takeno, A. Atlas of Eh-pH Diagrams: Intercomparison of Thermodynamic Databases; Geological Survey of Japan Open File Report No.419; National Institute of Advanced Industrial Science and Technology, Research Center for Deep Geological Environments: Tokyo, Japan, 2005; p. 285.
- Young, C.A.; Dahlgren, E.J.; Robins, R.G. The solubility of copper sulfides under reducing conditions. Hydrometallurgy 2003, 68, 23–31. [Google Scholar] [CrossRef]
- Ma, R.; Stegemeier, J.; Levard, C.; Dale, J.G.; Noack, C.W.; Yang, T.; Brown, G.E., Jr.; Lowry, G.V. Sulfidation of copper oxide nanoparticles and properties of resulting copper sulfide. Environ. Sci. Nano 2014, 1, 347–357. [Google Scholar] [CrossRef]





| # | Formula | Lower Limit of the Stability Field | Reference(s) |
|---|---|---|---|
| a | Cu4SnS4 | RT | [8,12,14,18,22,23] |
| b | Cu2SnS3 | RT | [8,12,13,18,22,23] |
| c | Cu2Sn4S9 | RT | [9,22,23] |
| Cu2Sn3S7 | [8,10,18,23] | ||
| Cu2Sn3.5S8 | [12,23] | ||
| Cu2Sn3.75S8 | [23] | ||
| Cu2Sn3.34S7.68 | [12] | ||
| Cu4Sn7S16 | [20,24] | ||
| d | Cu10Sn2S13 | 400 °C | [18,23] |
| Cu9.67Sn2.33S13 | [11,18,23] | ||
| Cu4SnS6 | [15,21,23] | ||
| e | Cu5Sn2S7 | 600 °C | [13,18,23] |
| f | Cu7Sn3S10 | 600 °C | [13,18,23] |
| g | Cu3SnS4 | RT * | [16,23] |
| Mineral | Log K | Reference |
|---|---|---|
| Cu3SnS4, kuramite | −73.14 | This study, wateq4f.dat [37] |
| Cu2SnS3, mohite | −58.03 | This study |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giaccherini, A.; Montegrossi, G.; Di Benedetto, F. Stability of Naturally Relevant Ternary Phases in the Cu–Sn–S System in Contact with an Aqueous Solution. Minerals 2016, 6, 79. https://doi.org/10.3390/min6030079
Giaccherini A, Montegrossi G, Di Benedetto F. Stability of Naturally Relevant Ternary Phases in the Cu–Sn–S System in Contact with an Aqueous Solution. Minerals. 2016; 6(3):79. https://doi.org/10.3390/min6030079
Chicago/Turabian StyleGiaccherini, Andrea, Giordano Montegrossi, and Francesco Di Benedetto. 2016. "Stability of Naturally Relevant Ternary Phases in the Cu–Sn–S System in Contact with an Aqueous Solution" Minerals 6, no. 3: 79. https://doi.org/10.3390/min6030079