5-Dodecylsalicylaldoxime as a Novel Collector in Cassiterite Flotation: Performance and Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Micro-Flotation Tests
2.2.2. Zeta Potential and Reagent Molecules Cluster Size Measurement
2.2.3. FTIR Analysis
2.2.4. XPS Analysis
3. Results and Discussions
3.1. Mineral Separation
3.1.1. Micro-Flotation of Single Mineral
3.1.2. Micro-Flotation of Artificial Mixed Ore
3.2. Mechanism of the Interaction between DSA and Minerals
3.2.1. Zeta Potential Measurements
3.2.2. FTIR Analysis
3.2.3. XPS Analysis
3.2.4. Reagent Molecules Cluster Size Measurement
3.2.5. Discussion of Adsorption Models
3.3. Production Application
4. Conclusions
- (1)
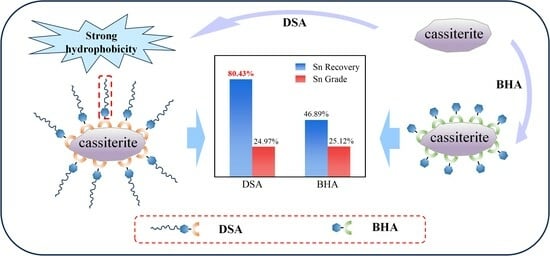
- In the micro-flotation test, the recovery of cassiterite reached an impressive 82.5% at a concentration of 9 × 10−5 mol/L, without the need for any activator. In the micro-flotation test of artificially mixed minerals, the concentrate with a Sn grade of 24.98% and a recovery of 80.44% was obtained at the same reagent concentration. Compared to BHA, while the Sn grade is similar, DSA achieved a 33.55% higher recovery.
- (2)
- Findings from zeta potential tests, FTIR, and XPS analyses indicate that DSA forms a stable chelating ring with the cassiterite surface, facilitated by the N and O atoms within its CH=NOH and C-OH groups. This adsorption mechanism resembles the process observed for BHA on cassiterite surfaces.
- (3)
- Analysis of reagent molecular cluster sizes reveals that DSA forms larger molecular clusters in the pulp and exhibits higher hydrophobicity than BHA. At equivalent active sites, DSA had a larger effective adsorption capacity than BHA, leading to enhanced hydrophobicity on the cassiterite surface and, thereby, favoring flotation recovery.
- (4)
- In a closed-circuit test of cassiterite flotation recovery in a copper–tin polymetallic ore in Yunnan, DSA achieved impressive results. It yielded a concentrate with a Sn grade of 14.751% and a recovery of 78.20% from raw ore with a Sn grade of 0.481%. Notably, this recovery surpassed that achieved with BHA as a collector by 12.29% while maintaining a nearly identical grade.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amosah, M.; Yvon, M.; Zhou, J.; Galvin, K. The role of enhanced desliming and gravity separation as a precursor to flotation in the upgrading of cassiterite from tailings. Miner. Eng. 2024, 208, 108581. [Google Scholar] [CrossRef]
- Yang, C.; Tan, Q.; Zeng, X.; Zhang, Y.; Wang, Z.; Li, J. Measuring the sustainability of tin in China. Sci. Total Environ. 2018, 635, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Kempf, A.; Kiefer, S.; Graczyk-Zajac, M.; Ionescu, E.; Riedel, R. Tin-functionalized silicon oxycarbide as a stable, high-capacity anode material for Na-ion batteries. Open Ceram. 2023, 15, 100388. [Google Scholar] [CrossRef]
- Izard, C.F.; Müller, D.B. Tracking the devil’s metal: Historical global and contemporary U.S. tin cycles. Resour. Conserv. Recycl. 2010, 54, 1436–1441. [Google Scholar] [CrossRef]
- Su, Z.; Zhang, Y.; Liu, B.; Lu, M.; Li, G.; Jiang, T. Extraction and Separation of Tin from Tin-Bearing Secondary Resources: A Review. JOM 2017, 69, 2364–2372. [Google Scholar] [CrossRef]
- Clauss, C.R.A.; Appleton, E.A.; Vink, J.J. Selective flocculation of cassiterite in mixtures with quartz using a modified polyacrylamide flocculant. Int. J. Miner. Process. 1976, 3, 27–34. [Google Scholar] [CrossRef]
- Angadi, S.I.; Eswaraiah, C.; Jeon, H.-S.; Mishra, B.K.; Miller, J.D. Selection of Gravity Separators for the Beneficiation of the Uljin Tin Ore. Miner. Process. Extr. Metall. Rev. 2017, 38, 54–61. [Google Scholar] [CrossRef]
- Zhang, L.; Khoso, S.; Tian, M.; Sun, W. Cassiterite recovery from a sulfide ore flotation tailing by combined gravity and flotation separations. Physicochem. Probl. Miner. Process. 2020, 57, 206–215. [Google Scholar] [CrossRef]
- Angadi, S.I.; Sreenivas, T.; Jeon, H.-S.; Baek, S.-H.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Miner. Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
- Leistner, T.; Embrechts, M.; Leißner, T.; Chehreh Chelgani, S.; Osbahr, I.; Möckel, R.; Peuker, U.A.; Rudolph, M. A study of the reprocessing of fine and ultrafine cassiterite from gravity tailing residues by using various flotation techniques. Miner. Eng. 2016, 96–97, 94–98. [Google Scholar] [CrossRef]
- Wang, X.; Liu, J.; Zhu, Y.; Li, Y. Adsorption and depression mechanism of an eco-friendly depressant PBTCA on fluorite surface for the efficient separation of cassiterite from fluorite. Miner. Eng. 2021, 171, 107124. [Google Scholar] [CrossRef]
- Peng, H.; Luo, W.; Wu, D.; Bie, X.; Shao, H.; Jiao, W.; Liu, Y. Study on the Effect of Fe3+ on Zircon Flotation Separation from Cassiterite Using Sodium Oleate as Collector. Minerals 2017, 7, 108. [Google Scholar] [CrossRef]
- Gruner, H.; Bilsing, U. Cassiterite flotation using styrene phosphonic acid to produce high-grade concentrates at high recoveries from finely disseminated ores-comparison with other collectors and discussion of effective circuit configurations. Miner. Eng. 1992, 5, 429–434. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Z.; Zeng, W.; Zhang, Y. Adhesion between nanobubbles and fine cassiterite particles. Int. J. Min. Sci. Technol. 2023, 33, 503–509. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, Y.; Qin, W.; Bao, S.; Wang, J. Collision and attachment behavior between fine cassiterite particles and H2 bubbles. Trans. Nonferrous Met. Soc. China 2014, 24, 520–527. [Google Scholar] [CrossRef]
- Chen, Y.; Tong, X.; Feng, D.; Xie, X. Effect of Al (III) Ions on the Separation of Cassiterite and Clinochlore Through Reverse Flotation. Minerals 2018, 8, 347. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, W. Surface Analysis of Cassiterite with Sodium Oleate in Aqueous Solution. Sep. Sci. Technol. 2012, 47, 502–506. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. Selective flotation of rare earth oxides from hematite and quartz mixtures using oleic acid as a collector. Int. J. Miner. Process. 2017, 169, 60–69. [Google Scholar] [CrossRef]
- Ding, Z.; Li, J.; Yuan, J.; Yu, A.; Wen, S.; Bai, S. Insights into the influence of calcium ions on the adsorption behavior of sodium oleate and its response to flotation of quartz: FT-IR, XPS and AMF studies. Miner. Eng. 2023, 204, 108437. [Google Scholar] [CrossRef]
- Pei, B.; Li, J.; Liu, Z.; Ning, S.; Cai, Z.; Liu, R. Reverse flotation separation of calcite/dolomite from hemimorphite based on their surface sulphophile and oxyphile affinity differences. Colloids Surf. A 2024, 682, 132932. [Google Scholar] [CrossRef]
- Liu, J.; Kong, D.; Xie, R.; Li, Y.; Zhu, Y.; Liu, C. Flotation behavior and mechanism of hydroxycitric acid as a depressant on the flotation separation of cassiterite from calcite. Miner. Eng. 2021, 170, 107046. [Google Scholar] [CrossRef]
- Li, F.; Zhong, H.; Zhao, G.; Wang, S.; Liu, G. Flotation performances and adsorption mechanism of α-hydroxyoctyl phosphinic acid to cassiterite. Appl. Surf. Sci. 2015, 353, 856–864. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, S.; Zhong, H. Optimization of conventional hydroxamic acid for cassiterite flotation: Application of structural modification under principle of isomerism. Miner. Eng. 2021, 167, 106901. [Google Scholar] [CrossRef]
- Sun, L.; Hu, Y.; Sun, W. Effect and mechanism of octanol in cassiterite flotation using benzohydroxamic acid as collector. Trans. Nonferrous Met. Soc. China 2016, 26, 3253–3257. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, J. Selective flotation of cassiterite with benzohyd roxamic acid. Miner. Eng. 2006, 19, 1410–1417. [Google Scholar] [CrossRef]
- Qin, W.; Xu, Y.; Liu, H.; Ren, L.; Yang, C. Flotation and Surface Behavior of Cassiterite with Salicylhydroxamic Acid. Ind. Eng. Chem. Res. 2011, 50, 10778–10783. [Google Scholar] [CrossRef]
- Cai, J.; Wu, B.; Deng, J.; Hu, M.; Wu, M.; Wei, P.; Sun, X.; Qiu, H.; Jin, X.; Hou, X.; et al. A novel approach to improve cassiterite recovery based on grinding. Powder Technol. 2022, 400, 117257. [Google Scholar] [CrossRef]
- Agrawal, Y.K.; Tandon, S.G. Solution stability constants of complexes of benzohydroxamic acid with some divalent metal ions. J. Inorg. Nucl. Chem. 1972, 34, 1291–1295. [Google Scholar] [CrossRef]
- Fu, J.; Han, H.; Wei, Z.; Liu, R.; Li, W.; Xu, T.; Ji, D. Selective separation of scheelite from calcite using tartaric acid and Pb-BHA complexes. Colloids Surf. A 2021, 622, 126657. [Google Scholar] [CrossRef]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids Surf. A 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, P.; Ou, L.; Zhang, Y.; Chen, J. Flotation of cassiterite using alkyl hydroxamates with different carbon chain lengths: A theoretical and experimental study. Miner. Eng. 2021, 170, 107025. [Google Scholar] [CrossRef]
- Houchin, M.R.; Warren, L.J. Surface titrations and electrokinetic measurements on Australian cassiterites. Colloids Surf. 1985, 16, 117–126. [Google Scholar] [CrossRef]
- Balachandran, S.B.; Simkovich, G.; Aplan, F.F. The influence of point defects on the floatability of cassiterite, I. Properties of synthetic and natural cassiterites. Int. J. Miner. Process. 1987, 21, 157–171. [Google Scholar] [CrossRef]
- Stachurski, J.; Michałek, M. The Effect of the ζ Potential on the Stability of a Non-Polar Oil-in-Water Emulsion. J. Colloid Interface Sci. 1996, 184, 433–436. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, J.; Zhu, Y.; Gong, G.; Han, Y. Mechanism of HCA and CEPPA in flotation separation of cassiterite and fluorite. Miner. Eng. 2022, 187, 107773. [Google Scholar] [CrossRef]
- Losos, Z.; Beran, A. OH defects in cassiterite. Mineral. Petrol. 2004, 81, 219–234. [Google Scholar] [CrossRef]
- Zheng, Z.P.; Fan, W.H.; Yan, H.; Liu, J.; Yang, W.Z.; Zhu, S.L. Terahertz and mid-infrared spectroscopy of benzene-1,2-diol. J. Mol. Spectrosc. 2012, 281, 13–17. [Google Scholar] [CrossRef]
- Keefe, C.D.; Gillis, E.A.L. Temperature dependence of the optical properties of liquid benzene in the infrared between 25 and 50 °C. Spectrochim. Acta Part A 2008, 70, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Nie, G.; Li, J.; Zhu, Z.; Wang, Z. Flotation separation of quartz and dolomite from collophane using sodium N-dodecyl-β-amino propionate and its adsorption mechanism. Colloids Surf. A 2022, 641, 128586. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, H.; Wang, S.; Niu, Y.; Liu, G. Synthesis of 2-ethyl-2-hexenal oxime and its flotation performance for copper ore. Miner. Eng. 2014, 66–68, 173–180. [Google Scholar] [CrossRef]
- Morozov, I.G.; Belousova, O.V.; Blanco-Andujar, C.; Ortega, D.; Kuznetsov, M.V. Structural, optical, magnetic, and XPS properties of SnOx nanoparticles. Solid State Sci. 2022, 126, 106854. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, L.; Gao, Z.; Sun, W.; Cao, X. Activation mechanism of zinc ions in cassiterite flotation with benzohydroxamic acid as a collector. Miner. Eng. 2020, 156, 106523. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, L.; Cao, Y.; Wang, X.; Qiao, Y.; Liu, G.; Xiang, M.; Sun, W. Flotation Separation of Smithsonite from Calcite Using Cupferron as a Collector. Minerals 2023, 13, 992. [Google Scholar] [CrossRef]
- Tian, M.; Liu, R.; Gao, Z.; Chen, P.; Han, H.; Wang, L.; Zhang, C.; Sun, W.; Hu, Y. Activation mechanism of Fe (III) ions in cassiterite flotation with benzohydroxamic acid collector. Miner. Eng. 2018, 119, 31–37. [Google Scholar] [CrossRef]












| Elements | Sn | Fe | O | Si | Ca | Al | Mg | Else |
|---|---|---|---|---|---|---|---|---|
| Content (wt %) | 0.481 | 2.593 | 51.00 | 41.12 | 0.24 | 1.76 | 1.14 | 1.6657 |
| Conditions | Products | Yield | Sn Grade | Sn Recovery |
|---|---|---|---|---|
| DSA | Concentrate | 2.55 | 14.751 | 78.20 |
| Tailings | 97.45 | 0.108 | 21.80 | |
| Raw ore | 100 | 0.480 | 100 | |
| BHA | Concentrate | 2.11 | 15.026 | 65.91 |
| Tailings | 97.89 | 0.167 | 34.09 | |
| Raw ore | 100 | 0.481 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Qiao, Y.; Cao, Y.; Wang, Q.; Wang, X.; Sun, W.; Liu, G. 5-Dodecylsalicylaldoxime as a Novel Collector in Cassiterite Flotation: Performance and Mechanism. Minerals 2024, 14, 190. https://doi.org/10.3390/min14020190
Sun L, Qiao Y, Cao Y, Wang Q, Wang X, Sun W, Liu G. 5-Dodecylsalicylaldoxime as a Novel Collector in Cassiterite Flotation: Performance and Mechanism. Minerals. 2024; 14(2):190. https://doi.org/10.3390/min14020190
Chicago/Turabian StyleSun, Lei, Yi Qiao, Yang Cao, Qingqing Wang, Xin Wang, Wei Sun, and Guobin Liu. 2024. "5-Dodecylsalicylaldoxime as a Novel Collector in Cassiterite Flotation: Performance and Mechanism" Minerals 14, no. 2: 190. https://doi.org/10.3390/min14020190