The Influence of Liquid/Solid Ratio and Pressure on the Natural and Accelerated Carbonation of Alkaline Wastes
Abstract
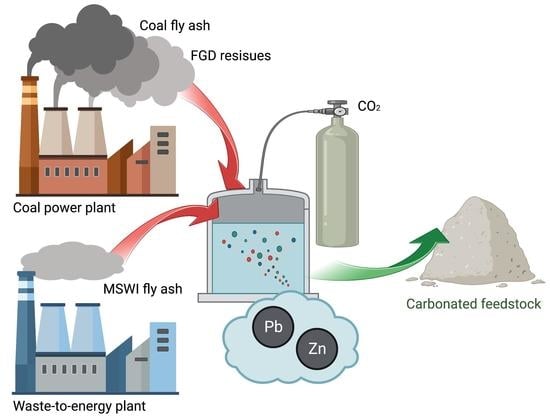
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Samples Preparation
2.2. Analytical Techniques
2.3. Carbonation Tests
3. Results and Discussion
3.1. Particle Size Distribution and Immediate Soluble Content
3.2. Ash Mineralogy
3.3. Natural and Accelerated Carbonation Experiments
3.3.1. Natural Carbonation Experiments
3.3.2. Accelerated Carbonation Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Press Office Climate Change Widespread, Rapid, and Intensifying—IPCC. Available online: https://www.ipcc.ch/2021/08/09/ar6-wg1-20210809-pr/#:~:text=The%20report%20shows%20that%20emissions,1.5%C2%B0C%20of%20warming (accessed on 26 June 2023).
- Al-Ghussain, L. Global warming: Review on driving forces and mitigation. Environ. Prog. Sustain. Energy 2019, 38, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Carleton, T.A.; Hsiang, S.M. Social and economic impacts of climate. Science 2016, 353, aad9837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shayanmehr, S.; Rastegari Henneberry, S.; Sabouhi Sabouni, M.; Shahnoushi Foroushani, N. Climate Change and Sustainability of Crop Yield in Dry Regions Food Insecurity. Sustainability 2020, 12, 9890. [Google Scholar] [CrossRef]
- Berchin, I.I.; Valduga, I.B.; Garcia, J.; de Andrade Guerra, J.B.S.O. Climate change and forced migrations: An effort towards recognizing climate refugees. Geoforum 2017, 84, 147–150. [Google Scholar] [CrossRef]
- Otto, A.; Robinius, M.; Grube, T.; Schiebahn, S.; Praktiknjo, A.; Stolten, D. Power-to-Steel: Reducing CO2 through the Integration of Renewable Energy and Hydrogen into the German Steel Industry. Energies 2017, 10, 451. [Google Scholar] [CrossRef] [Green Version]
- Elavarasan, R.M.; Afridhis, S.; Vijayaraghavan, R.R.; Subramaniam, U.; Nurunnabi, M. SWOT analysis: A framework for comprehensive evaluation of drivers and barriers for renewable energy development in significant countries. Energy Rep. 2020, 6, 1838–1864. [Google Scholar] [CrossRef]
- Socolow, R.; Hotinski, R.; Greenblatt, J.B.; Pacala, S. Solving the Climate Problem: Technologies Available to Curb CO2 Emissions. Environ. Sci. Policy Sustain. Dev. 2004, 46, 8–19. [Google Scholar] [CrossRef]
- Songolzadeh, M.; Soleimani, M.; Ravanchi, M.T.; Songolzadeh, R. Carbon Dioxide Separation from Flue Gases: A Technological Review Emphasizing Reduction in Greenhouse Gas Emissions. Sci. World J. 2014, 2014, 828131. [Google Scholar] [CrossRef] [Green Version]
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef] [Green Version]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnabel, K.; Brück, F.; Pohl, S.; Mansfeldt, T.; Weigand, H. Technically exploitable mineral carbonation potential of four alkaline waste materials and effects on contaminant mobility. Greenh. Gases Sci. Technol. 2021, 11, 506–519. [Google Scholar] [CrossRef]
- Bobicki, E.R.; Liu, Q.; Xu, Z.; Zeng, H. Carbon capture and storage using alkaline industrial wastes. Prog. Energy Combust. Sci. 2012, 38, 302–320. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Liu, K.; Huang, D.-M.; Chen, Q.; Tu, M.-J.; Wu, K.; Shui, Z.-H. Utilization of fly ash as building material admixture: Basic properties and heavy metal leaching. Case Stud. Constr. Mater. 2022, 17, e01422. [Google Scholar] [CrossRef]
- Bauer, M.; Gassen, N.; Stanjek, H.; Peiffer, S. Carbonation of lignite fly ash at ambient T and P in a semi-dry reaction system for CO2 sequestration. Appl. Geochem. 2011, 26, 1502–1512. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Yu, B.; Zhang, R.; French, D.; Grigore, M.; Wang, X.; Chen, Z.; Zhao, S. Insights into Carbonation Kinetics of Fly Ash from Victorian Lignite for CO2 Sequestration. Energy Fuels 2018, 32, 4569–4578. [Google Scholar] [CrossRef]
- Costa, G.; Baciocchi, R.; Polettini, A.; Pomi, R.; Hills, C.D.; Carey, P.J. Current status and perspectives of accelerated carbonation processes on municipal waste combustion residues. Environ. Monit. Assess. 2007, 135, 55–75. [Google Scholar] [CrossRef]
- Ji, L.; Yu, H.; Zhang, R.; French, D.; Grigore, M.; Yu, B.; Wang, X.; Yu, J.; Zhao, S. Effects of fly ash properties on carbonation efficiency in CO2 mineralisation. Fuel Process. Technol. 2019, 188, 79–88. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Z.; Cheng, H.; Zhang, Z.; Cheng, F. A review of carbon dioxide sequestration by mineral carbonation of industrial byproduct gypsum. J. Clean. Prod. 2021, 302, 126930. [Google Scholar] [CrossRef]
- Montes-Hernandez, G.; Pérez-López, R.; Renard, F.; Nieto, J.M.; Charlet, L. Mineral sequestration of CO2 by aqueous carbonation of coal combustion fly-ash. J. Hazard. Mater. 2009, 161, 1347–1354. [Google Scholar] [CrossRef] [Green Version]
- Kuusik, R.; Uibu, M.; Kirsimäe, K.; Mõtlep, R.; Meriste, T. Open-air deposition of estonian oil shale ash: Formation, state of art, problems and prospects for the abatement of environmental impact. Oil Shale 2012, 29, 376. [Google Scholar] [CrossRef] [Green Version]
- Berber, H.; Tamm, K.; Leinus, M.-L.; Kuusik, R.; Tõnsuaadu, K.; Paaver, P.; Uibu, M. Accelerated Carbonation Technology Granulation of Industrial Waste: Effects of Mixture Composition on Product Properties. Waste Manag. Res. 2020, 38, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Assi, A.; Federici, S.; Bilo, F.; Zacco, A.; Depero, L.E.; Bontempi, E. Increased Sustainability of Carbon Dioxide Mineral Sequestration by a Technology Involving Fly Ash Stabilization. Materials 2019, 12, 2714. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Regnault, O.; Lagneau, V.; Schneider, H. Experimental measurement of portlandite carbonation kinetics with supercritical CO2. Chem. Geol. 2009, 265, 113–121. [Google Scholar] [CrossRef]
- Sorrentino, G.P.; Zanoletti, A.; Ducoli, S.; Zacco, A.; Iora, P.; Invernizzi, C.M.; Di Marcoberardino, G.; Depero, L.E.; Bontempi, E. Accelerated and natural carbonation of a municipal solid waste incineration (MSWI) fly ash mixture: Basic strategies for higher carbon dioxide sequestration and reliable mass quantification. Environ. Res. 2023, 217, 114805. [Google Scholar] [CrossRef]
- Ramezanianpour, A.A. Cement Replacement Materials; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-36720-5. [Google Scholar]
- Bontempi, E.; Zacco, A.; Borgese, L.; Gianoncelli, A.; Ardesi, R.; Depero, L.E. A new method for municipal solid waste incinerator (MSWI) fly ash inertization, based on colloidal silica. J. Environ. Monit. 2010, 12, 2093–2099. [Google Scholar] [CrossRef]
- Pandey, R.A.; Biswas, R.; Chakrabarti, T.; Devotta, S. Flue Gas Desulfurization: Physicochemical and Biotechnological Approaches. Crit. Rev. Environ. Sci. Technol. 2005, 35, 571–622. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Federici, S.; Zacco, A.; Depero, L.E.; Bontempi, E. Bottom ash derived from municipal solid waste and sewage sludge co-incineration: First results about characterization and reuse. Waste Manag. 2020, 116, 147–156. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Borgese, L.; Depero, L.E.; Nenci, M.; Bontempi, E. Stabilization of Municipal Solid Waste Fly Ash, Obtained by Co-Combustion with Sewage Sludge, Mixed with Bottom Ash Derived by the Same Plant. Appl. Sci. 2020, 10, 6075. [Google Scholar] [CrossRef]
- Assi, A.; Bilo, F.; Zanoletti, A.; Ponti, J.; Valsesia, A.; La Spina, R.; Zacco, A.; Bontempi, E. Zero-waste approach in municipal solid waste incineration: Reuse of bottom ash to stabilize fly ash. J. Clean. Prod. 2019, 245, 118779. [Google Scholar] [CrossRef]
- ISO 7404–2; Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples. International Organization for Standardization: Geneva, Switzerland, 2009.
- DS/EN 15149-2; Solid Biofuels—Determination of Particle Size Distribution—Part 2: Vibrating Screen Method Using Sieve Apertures of 3.15 mm and Below. Danish Standards Foundation: Copenhagen, Denmark, 2010.
- ASTM C25-19; Standard Test Methods for Chemical Analysis of Limestone, Quicklime, and Hydrated Lime. ASTM: West Conshohocken, PA, USA, 2019. [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef] [Green Version]
- Black, L. Low Clinker Cement as a Sustainable Construction Material. In Sustainability of Construction Materials; Elsevier: Amsterdam, The Netherlands, 2016; pp. 415–457. [Google Scholar] [CrossRef]
- Walker, R.; Pavía, S. Physical properties and reactivity of pozzolans, and their influence on the properties of lime–pozzolan pastes. Mater. Struct. 2010, 44, 1139–1150. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Pan, S.-Y.; Chang, E.-E.; Kim, H.; Chen, Y.-H.; Chiang, P.-C. Validating carbonation parameters of alkaline solid wastes via integrated thermal analyses: Principles and applications. J. Hazard. Mater. 2016, 307, 253–262. [Google Scholar] [CrossRef]
- Gu, Q.; Wang, T.; Wu, W.; Wang, D.; Jin, B. Influence of pretreatments on accelerated dry carbonation of MSWI fly ash under medium temperatures. Chem. Eng. J. 2021, 414, 128756. [Google Scholar] [CrossRef]
- Wang, L.; Jin, Y.; Nie, Y. Investigation of accelerated and natural carbonation of MSWI fly ash with a high content of Ca. J. Hazard. Mater. 2010, 174, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Hover, K.C. The influence of water on the performance of concrete. Constr. Build. Mater. 2011, 25, 3003–3013. [Google Scholar] [CrossRef]
- Brück, F.; Schnabel, K.; Mansfeldt, T.; Weigand, H. Accelerated carbonation of waste incinerator bottom ash in a rotating drum batch reactor. J. Environ. Chem. Eng. 2018, 6, 5259–5268. [Google Scholar] [CrossRef]
- Oral, Ç.M.; Ercan, B. Influence of pH on morphology, size and polymorph of room temperature synthesized calcium carbonate particles. Powder Technol. 2018, 339, 781–788. [Google Scholar] [CrossRef]
- Black, L.; Breen, C.; Yarwood, J.; Garbev, K.; Stemmermann, P.; Gasharova, B. Structural Features of C–S–H(I) and Its Carbonation in Air? A Raman Spectroscopic Study. Part II: Carbonated Phases. J. Am. Ceram. Soc. 2007, 90, 908–917. [Google Scholar] [CrossRef]
- Sarkar, A.; Mahapatra, S. Synthesis of All Crystalline Phases of Anhydrous Calcium Carbonate. Cryst. Growth Des. 2010, 10, 2129–2135. [Google Scholar] [CrossRef]
- Ouhadi, V.; Yong, R.N.; Amiri, M.; Ouhadi, M. Pozzolanic consolidation of stabilized soft clays. Appl. Clay Sci. 2014, 95, 111–118. [Google Scholar] [CrossRef]
- Thomas, M.; Jewell, R.; Jones, R. Coal Fly Ash as a Pozzolan. In Coal Combustion Products (CCP’s); Elsevier: Amsterdam, The Netherlands, 2017; pp. 121–154. [Google Scholar]
- Slegers, P.A.; Rouxhet, P.G. Carbonation of the hydration products of tricalcium silicate. Cem. Concr. Res. 1976, 6, 381–388. [Google Scholar] [CrossRef]







| Elements | CFA | MSWI FA | FGD | MSWI BA |
|---|---|---|---|---|
| mg/kg | mg/kg | mg/kg | mg/kg | |
| P * | 690 ± 230 | 2000 ± 100 | 1000 ± 400 | 3960 ± 460 |
| S * | 5180 ± 1160 | 13,100 ± 900 | 54,400 ± 9900 | 1590 ± 200 |
| Cl * | 900 ± 187 | 1500 ± 400 | 80 ± 10 | 3700 ± 660 |
| K | 6360 ± 1118 | 24,610 ± 1700 | 2000 ± 270 | 8520 ± 310 |
| Ca | 4180 ± 916 | 69,200 ± 3100 | 38,300 ± 3380 | 79,100 ± 7400 |
| Fe | 21,500 ± 700 | 3750 ± 170 | 160 ± 30 | 28,820 ± 9320 |
| Cu | 710 ± 30 | 710 ± 20 | 2050 ± 120 | 2420 ± 80 |
| Zn | 2440 ± 130 | 8800 ± 200 | 2920 ± 250 | 4280 ± 120 |
| Pb | <LOD | 1030 ± 50 | <LOD | 3740 ± 200 |
| Samples | BLEND | NC1_0.7 | NC1_0.9 | NC2_0.9 | NC1_1.2 | AC_0.7 | AC_0.9 | AC_0.9_bis | AC_1.2 |
|---|---|---|---|---|---|---|---|---|---|
| Carbonation type | NC | NC | NC | NC | AC | AC | AC | AC | |
| L/S | 0.7 | 0.9 | 0.9 | 1.2 | 0.7 | 0.9 | 0.9 | 1.2 | |
| Time test | 1 month | 1 month | 2 months | 1 month | 17 h | 72 h | 12 days | 51 h |
| Sample | Process | Size Fractions (μm) | ||||||
|---|---|---|---|---|---|---|---|---|
| >150 | 75–150 | 45–75 | 25–45 | <25 | ||||
| Dry sieving (DS) | wt.% | |||||||
| CFA | 4.3 | 8.9 | 12.6 | 34.7 | 39.6 | |||
| MSWI FA | 8.0 | 9.2 | 11.8 | 22.1 | 48.9 | |||
| FGD | 2.5 | 8.6 | 28.2 | 57.1 | 4.6 | |||
| Wet sieving (WS) | wt.% | |||||||
| CFA | 3.2 | 7.3 | 11.0 | 16.3 | 54.4 | |||
| MSWI FA | 6.9 | 5.7 | 4.4 | 4.5 | 31.5 | |||
| FGD | 0.3 | 5.1 | 4.2 | 4.7 | 57.2 | |||
| >4000 | 2000–4000 | 1000–2000 | 500–1000 | 250–500 | 150–250 | <150 | ||
| Dry sieving (DS) | wt.% | |||||||
| MSWI BA | 41.1 | 17.8 | 17.6 | 9.5 | 5.1 | 2.6 | 6.3 | |
| Samples | CFA | MSWI FA | FGD | MSWI BA <150 μm | MSWI BA <2 mm Ground |
|---|---|---|---|---|---|
| wt.% | |||||
| Calcium hydroxy chloride (CaClOH) | <1 | 26 | <1 | <1 | <1 |
| Portlandite (Ca(OH)2) | <1 | 4 | 23 | <1 | <1 |
| Calcite (CaCO3) | <1 | 9 | 11 | 14 | 7 |
| Anhydrite (CaSO4) | <1 | 4 | <1 | 1 | <1 |
| Hannebachite (CaSO3 *0.5 H2O) | <1 | <1 | 21 | <1 | <1 |
| Bassanite (CaSO4 *0.5 H2O) | <1 | 4 | 7 | <1 | <1 |
| Gypsum (CaSO4 *2 H2O) | <1 | 2 | 4 | <1 | <1 |
| Quartz (SiO2) | 21 | <1 | <1 | 5 | 9 |
| Mullite (Al6Si2O13) | 17 | <1 | <1 | <1 | <1 |
| Hematite (Fe2O3) | 1 | <1 | <1 | 1 | <1 |
| Tripotassium phosphate (K3PO4) | <1 | 3 | <1 | 5 | 8 |
| Halite (NaCl) | <1 | 5 | <1 | <1 | <1 |
| Sylvite (KCl) | <1 | 3 | <1 | <1 | <1 |
| Amorphous | 60 | 41 | 33 | 72 | 76 |
| Samples | BLEND | NC1_0.7 | NC1_0.9 | NC1_1.2 | AC_0.7 | AC_0.9 | AC_0.9_bis | AC_1.2 |
|---|---|---|---|---|---|---|---|---|
| wt.% | ||||||||
| LOI (1000 °C) | 24.21 | 30.56 | 31.31 | 31.04 | 26.92 | 27.83 | 28.99 | |
| Samples | BLEND | NC1_0.7 | NC1_0.9 | NC1_1.2 | AC_0.7 | AC_0.9 | AC_0.9_bis | AC_1.2 |
|---|---|---|---|---|---|---|---|---|
| wt.% | ||||||||
| Calcium hydroxy chloride (CaClOH) | 16 | <1 | <1 | <1 | 9 | 8 | 3 | 7 |
| Portlandite (Ca(OH)2) | 7 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Calcite (CaCO3) | 10 | 28 | 32 | 28 | 12 | 20 | 24 | 27 |
| Vaterite (CaCO3) | <1 | 5 | 2 | 3 | 5 | 4 | 6 | 2 |
| Anhydrite (CaSO4) | 3 | 2 | 2 | 2 | 5 | 2 | 1 | 2 |
| Hannebachite (CaSO3 *0.5 H2O) | 4 | 3 | 4 | 4 | 6 | 4 | 6 | 4 |
| Bassanite (CaSO4 *0.5 H2O) | 3 | 5 | 3 | 5 | 3 | 2 | 3 | 4 |
| Gypsum (CaSO4 *2 H2O) | 3 | <1 | 1 | 1 | 1 | 2 | 1 | < 1 |
| Quartz (SiO2) | 5 | 4 | 3 | 4 | 3 | 5 | 3 | 4 |
| Hematite (Fe2O3) | 1 | <1 | <1 | <1 | 1 | 1 | <1 | <1 |
| Tripotassium phosphate (K3PO4) | 1 | 2 | 2 | 1 | 3 | 2 | 2 | 1 |
| Halite (NaCl) | 3 | 3 | 2 | 2 | 2 | 2 | 2 | 3 |
| Sylvite (KCl) | 2 | <1 | 2 | 1 | 1 | 2 | 1 | < 1 |
| Amorphous | 42 | 45 | 45 | 48 | 47 | 45 | 47 | 42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrentino, G.P.; Guimarães, R.; Valentim, B.; Bontempi, E. The Influence of Liquid/Solid Ratio and Pressure on the Natural and Accelerated Carbonation of Alkaline Wastes. Minerals 2023, 13, 1060. https://doi.org/10.3390/min13081060
Sorrentino GP, Guimarães R, Valentim B, Bontempi E. The Influence of Liquid/Solid Ratio and Pressure on the Natural and Accelerated Carbonation of Alkaline Wastes. Minerals. 2023; 13(8):1060. https://doi.org/10.3390/min13081060
Chicago/Turabian StyleSorrentino, Giampiero Pasquale, Renato Guimarães, Bruno Valentim, and Elza Bontempi. 2023. "The Influence of Liquid/Solid Ratio and Pressure on the Natural and Accelerated Carbonation of Alkaline Wastes" Minerals 13, no. 8: 1060. https://doi.org/10.3390/min13081060