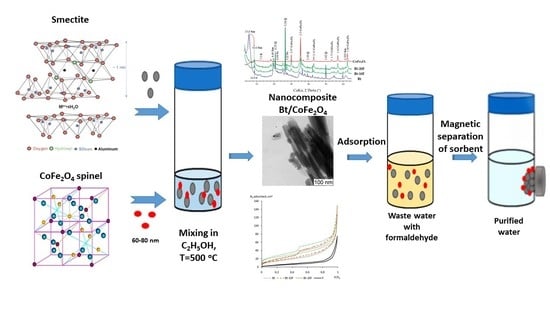
Magnetic Nanosorbents Based on Bentonite and CoFe2O4 Spinel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Cobalt Ferrite CoFe2O4
2.2. Fabrication of Nanocomposites
2.3. Methods for Composition Diagnostics
2.4. Methods for the Properties Analysis
3. Results and Discussion
3.1. Characterization of Composition and Structure of CoFe2O4, Natural Aluminosilicate and Composites on Their Base
3.2. Characterization of the Sorption Ability of Ferrite, Fe-Smectite and Composites on Their Base
3.3. Magnetic Properties of Bentonite, CoFe2O4 Spinel and Composites on Their Base
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paris, E.; Malafatti, J.; Musetti, H.; Manzoli, A.; Zenatti, A.; Escote, M. Faujasite zeolite decorated with cobalt ferrite nanoparticles for improving removal and reuse in Pb2+ ions adsorption. Chin. J. Chem. Eng. 2020, 28, 1884–1890. [Google Scholar] [CrossRef]
- Suppaso, C.; Pongkan, N.; Intachai, S.; Ogawa, M.; Khaorapapong, N. Magnetically recoverable β-Ni(OH)2/γ-Fe2O3/NiFe-LDH composites; isotherm, thermodynamic and kinetic studies of synthetic dye adsorption and photocatalytic activity. Appl. Clay Sci. 2021, 213, 106115. [Google Scholar] [CrossRef]
- Agboola, O.; Popoola, P.; Sadiku, R.; Sanni, S.E.; Fayomi, S.O.; Fatoba, O.S. Nanotechnology in Wastewater and the Capacity of Nanotechnology for Sustainability. In Environmental Nanotechnology Volume 3. Environmental Chemistry for a Sustainable World; Dasgupta, N., Ranjan, S., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2020; Volume 27, pp. 1–45. [Google Scholar]
- Sahu, J.; Karri, R.R.; Zabed, H.M.; Shams, S.; Qi, X. Current perspectives and future prospects of nano-biotechnology in wastewater treatment. Sep. Purif. Rev. 2021, 50, 139–158. [Google Scholar] [CrossRef]
- Guo, W.; Fu, Z.; Zhang, Z.; Wang, H.; Liu, S.; Feng, W.; Zhao, X.; Giesy, J.P. Synthesis of Fe3O4 magnetic nanoparticles coated with cationic surfactants and their applications in Sb (V) removal from water. Sci. Total Environ. 2020, 710, 136302. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Dethe, M.J.; Marathe, K.V.; Gaikar, V.G. Adsorption of Lactic Acid on Weak Base Polymeric Resins. Sep. Sci. Technol. 2006, 41, 2947–2971. [Google Scholar] [CrossRef]
- Hall, S.; Tang, R.; Baeyens, J.; Dewil, R. Removing polycyclic aromatic hydrocarbons from water by adsorption on silicagel. Polycycl. Aromat. Compd. 2009, 29, 160–183. [Google Scholar] [CrossRef]
- Koster, H.M.; Ehrlicher, U.; Gilg, H.; Jordan, R.; Murad, E.; Onnich, K. Mineralogical and chemical characteristics of five nontronites and Fe-rich smectite. Clay Miner. 1999, 34, 579–599. [Google Scholar] [CrossRef]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
- Abidi, N.; Errais, E.; Duplay, J.; Berez, A.; Jrad, A.; Schäfer, G.; Ghazi, M.; Semhi, K.; Trabelsi-Ayadi, M. Treatment of dye-containing effluent by natural clay. J. Clean. Prod. 2015, 86, 432–440. [Google Scholar] [CrossRef]
- Juang, R.-S.; Lin, S.-H.; Tsao, K.-H. Mechanism of the Sorption of Phenols from Aqueous Solutions into Surfactant-Modified Montmorillonite. J. Colloid Interface Sci. 2002, 254, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Bel’chinskaya, L.I.; Khodosova, N.A.; Novikova, L.A.; Anisimov, M.V.; Petukhova, G.A. Regulation of sorption processes in natural nanoporousaluminosilicates. 3. Impact of electromagnetic fields on adsorption and desorption of formaldehyde by clinoptilolite. Prot. Met. Phys. Chem. Surf. 2017, 53, 793–800. [Google Scholar] [CrossRef]
- Bel’chinskaya, L.I.; Khodosova, N.A.; Novikova, L.A.; Strel’nikova, O.Y.; Roessner, F.; Petukhova, G.A.; Zhabin, A.V. Regulation of sorption processes in natural nanoporous aluminosilicates. 2. Determination of the ratio between active sites. Prot. Met. Phys. Chem. Surf. 2016, 52, 599–606. [Google Scholar] [CrossRef]
- Gubin, S.P.; Koksharov, Y.A.; Khomutov, G.B.; Yurkov, G.Y. Magnetic nanoparticles: Preparation, structure and properties. Russ. Chem. Rev. 2005, 74, 539–574. [Google Scholar] [CrossRef]
- Lu, A.-H.; Salabas, E.L.; Schuth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef]
- Soltani, N.; Salavati, H.; Moghadasi, A. The role of Na-montmorillonite/cobalt ferrite nanoparticles in the corrosion of epoxy coated AA 3105 aluminum alloy. Surf. Interfaces 2019, 15, 89–99. [Google Scholar] [CrossRef]
- Aguiara, A.S.; Michels, L.; da Silva, F.G.; Kernc, C.; Gomide, G.; Ferreira, C.M.; Depeyrot, J.; Aquino, R.; da Silva, G.J. The use of a laponite dispersion to increase the hydrophilicity of cobalt ferrite magnetic nanoparticles. Appl. Clay Sci. 2020, 193, 105663. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Abdelwahab, M.S.; Fathallah, E.M. Design of novel nano-sorbents based on nano-magnetic iron oxide–bound-nano-silicon oxide–immobilized-triethylenetetramine for implementation in water treatment of heavy metals. Chem. Eng. J. 2013, 223, 318–327. [Google Scholar] [CrossRef]
- Haicheng, L.; Wei, C.; Cheng, L.; Yu, L.; Changlong, D. Magnetic mesoporous clay adsorbent: Preparation, characterization and adsorption capacity for atrazine. Microporous Mesoporous Mater. 2014, 194, 72–78. [Google Scholar]
- Lutfullin, M.A.; Shornikova, O.N.; Vasiliev, A.V.; Pokholok, K.V.; Osadchaya, V.A.; Saidaminov, M.I.; Sorokina, N.E.; Avdeev, V.V. Petroleum products and water sorption by expanded graphite enhanced with magnetic iron phases. Carbon 2014, 66, 417–425. [Google Scholar] [CrossRef]
- Tkachenko, I.A.; Panasenko, A.E.; Odinokov, M.M.; Marchenko, Y.V. Magnetoactive Composite Sorbents CoFe2O4–SiO2. Russ. J. Inorg. Chem. 2020, 65, 1142–1149. [Google Scholar] [CrossRef]
- Tolmacheva, V.V.; Apyari, V.V.; Kochuk, E.V.; Dmitrenko, S.G. Magnetic adsorbents based on iron oxide nanoparticles for the extraction and preconcentration of organic compounds. J. Anal. Chem. 2016, 71, 339–356. [Google Scholar] [CrossRef]
- Germanov, E.P.; Kutuschov, M.V. Magnetically Controlled Sorbent and a Method for Manufacture Thereof (Russia, Patent RU 2 255 800 C1) Rospatent. 2003. Available online: https://rospatent.gov.ru (accessed on 10 July 2005).
- Kharisov, B.I.; Dias, V.R.; Kharissova, O.V. Mini-review: Ferrite nanoparticles in the catalysis. Arab. J. Chem. 2014, 104, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Agafonov, A.V.; Grishina, E.P. Nanocomposites of Inorganic Oxides with Ionic Liquids. Synthesis, Properties, Application (Review). Russ. J. Inorg. Chem. 2019, 64, 1641–1648. [Google Scholar] [CrossRef]
- Ali, I.; ALOthman, Z.A.; Sanagi, M.M. Green Synthesis of iron nano-impregnated adsorbent for fast removal of fluoride from Water. J. Mol. Liq. 2015, 211, 457–465. [Google Scholar] [CrossRef]
- Cui, L.; Wang, Y.; Gao, L.; Hu, L.; Yan, L.; Wei, Q.; Du, B. EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: Adsorption mechanism and separation property. Chem. Eng. J. 2015, 28, 1–10. [Google Scholar] [CrossRef]
- Ali, I.; Alothman, Z.A.; Al-Warthan, A. Synthesis of composite iron nano adsorbent and removal of ibuprofen drug residue from water. J. Mol. Liq. 2016, 219, 858–864. [Google Scholar] [CrossRef]
- Roshanfekr Rad, L.; Anbia, M. Zeolite-based composites for the adsorption of toxic matters from water: A review. J. Environ. Chem. Eng. 2021, 9, 106088. [Google Scholar] [CrossRef]
- Zhang, J.; Khan, M.A.; Xia, M.; Abdo, A.M.; Lei, W.; Liao, C.; Wang, F. Facile hydrothermal synthesis of magnetic adsorbent CoFe2O4/MMT to eliminate antibiotics in aqueous phase: Tetracycline and ciprofloxacin. Environ. Sci. Pollut. Res. 2019, 26, 215–226. [Google Scholar] [CrossRef]
- Vidal, C.B.; Santos, B.A.; França, A.M.; Bessa, R.A.; Loiola, A.R.; Nascimento, R.F. Magnetite-Zeolite Nanocomposite Applied to Remediation of Polluted Aquatic Environments. In Nanomaterials and Nanotechnology; Nascimento, R.F., Sousa Neto, V.O., Almeida Fechine, P.B., Cavalcante Freire, P.T., Eds.; Springer: Singapore, 2021; pp. 69–94. [Google Scholar]
- Reddy, D.H.K.; Yun, Y.-S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Shapkin, N.P.; Khal’chenko, I.G.; Pechnikov, V.S.; Mayorov, V.Y.; Maslova, N.V.; Razov, V.I.; Papynov, E.K. Magnetic Composites Based on Cobalt Ferrite, Vermiculite, and Rice Husks: Synthesis and Properties. Russ. J. Inorg. Chem. 2020, 65, 1614–1622. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Thorat, N.D.; Phadatare, M.R.; Sathish, C.I.; Dhawale, D.S.; Pawar, S.H. Polyvinyl alcohol functionalized cobalt ferrite nanoparticles for biomedical applications. Appl. Surf. Sci. 2013, 264, 598–604. [Google Scholar] [CrossRef]
- Liu, F.; Laurent, S.; Fattahi, H.; Vander Elst, L.; Muller, R.N. Superparamagnetic nanosystems based on iron oxide nanoparticles for biomedical imaging. Nanomedicine 2011, 6, 519–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, E.; Kotsikau, D.; Pankov, V.; Fahmi, A. Influence of Synthesis Methods on Structural and Magnetic Characteristics of Mg–Zn-Ferrite Nanopowders. J. Magn. Magn. Mater. 2019, 473, 85–91. [Google Scholar] [CrossRef]
- Somnath, S.; Indu, S.; Kotnala, R.K.; Singh, M.; Kumar, A.; Dhiman, P.; Singh, V.P.; Verma, K.; Kumar, G. Structural Magnetic and Mössbauer Studies of Nd-Doped Mg-Mn Ferrite Nanoparticles. J. Magn. Magn. Mater. 2017, 444, 77–86. [Google Scholar] [CrossRef]
- Manikandan, A.; Sridhar, R.; Antony, S.A.; Ramakrishna, S. A Simple Aloe Vera Plant-Extracted Microwave and Conventional Combustion Synthesis: Morphological, Optical, Magnetic and Catalytic Properties of CoFe2O4 Nanostructures. J. Mol. Struct. 2014, 1076, 188–200. [Google Scholar] [CrossRef]
- Song, Q.; Zhang, Z.J. Shape Control and Associated Magnetic Properties of Spinel Cobalt Ferrite Nanocrystals. J. Am. Chem. Soc. 2004, 126, 6164–6168. [Google Scholar] [CrossRef]
- Pallai, V.; Shah, D.O. Synthesis of High-Coercivity Cobalt Ferrite Particles Using Water-in-Oil Microemulsions. J. Magn. Magn. Mater. 1996, 163, 243–248. [Google Scholar] [CrossRef]
- Skomski, R. Nanomagnetics. J. Phys. Condens. Matter. 2003, 15, 841–896. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, C.H.; Fiore, S.; Tong, D.S.; Zhang, H.; Li, C.S.; Ji, S.F.; Yu, W.H. Functional magnetic nanoparticle/clay mineral nanocomposites: Preparation, magnetism and versatile applications. Appl. Clay Sci. 2016, 127–128, 143–163. [Google Scholar] [CrossRef]
- Zhang, S.; Zhong, L.; Yang, H.; Tang, A.; Zuo, X. Magnetic carbon-coated palygorskite loaded with cobalt nanoparticles for Congo Red removal from waters. Appl. Clay Sci. 2020, 198, 105856. [Google Scholar] [CrossRef]
- Palza, H.; Delgado, K.; Govan, J. Novel magnetic CoFe2O4/layered double hydroxide nanocomposites for recoverable anionic adsorbents for water treatment. Appl. Clay Sci. 2019, 183, 105350. [Google Scholar] [CrossRef]
- Khodosova, N.A.; Tomina, E.V.; Belchinskaya, L.I.; Zhabin, A.V.; Volkov, A.S.; Kurkin, N.A. Physical and Chemical Characteristics Of A Nanocomposite Sorbent, Nontronite/CoFe2O4. J. Sorp. Chromatogr. Proces. 2021, 24, 520–528. [Google Scholar]
- Bartenev, V.K.; Savko, A.D. Lithology, facies and minerals of the Paleogene of the TsChER. In Proceedings of the Scientific Research Institute of Geology of the Voronezh State University; SINOAL: Voronezh, Russia, 2001; Volume 7, 146p. [Google Scholar]
- Shashank, D.B.; Rakesh, K.S.; Vivek, K.; Nishant, K.; Shambhu, K. Tailoring the structural, optical and multiferroic properties of low temperature synthesized cobalt ferrite nanomaterials, by citrate precursor method. Mater. Today Proc. 2021, 46, 6527–6533. [Google Scholar]
- Ali, T.M.; Ismail, S.M.; Mansour, S.F.; Abdo, M.A.; Yehia, M. Physical properties of Al-doped cobalt nanoferrite prepared by citrate–nitrate auto combustion method. J. Mater. Sci. Mater. Electron. 2021, 32, 3092–3103. [Google Scholar] [CrossRef]
- Mariosi, F.R.; Venturini, J.; Alexandre, C.V.; Bergmann, C.P. Lanthanum-doped spinel cobalt ferrite (CoFe2O4) nanoparticles for environmental applications. Ceram. Int. 2019, 46, 2772–2779. [Google Scholar] [CrossRef]
- JCPDC PCPDFWIN: A Windows Retrieval/Display Program for Accessing the ICDD PDF—2 Data Base; International Centre for Diffraction Data: Newtown Square, PA, USA, 1997.
- Subasi, N.T. Formaldehyde Advantages and Disadvantages: Usage Areas and Harmful Effects on Human Beings. In Bio-Chemical Toxicology—Heavy Metals and Nanomaterials; Ince, M., Ince, O.K., Ondrasek, G., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Gates, W.P. Infrared spectroscopy and the chemistry of dioctahedral smectites. In Vibrational Spectroscopy of Layer Silicates and Hydroxides, 1st ed.; Klopproge, J.T., Ed.; The Clay Mineral Society: Aurora, CO, USA, 2005; pp. 126–168. [Google Scholar]
- Rojas-Mantilla, H.D.; Ayala-Duran, S.C.; Pupo Nogueira, R.F. Nontronite mineral clay NAu-2 as support for hematite applied as catalyst for heterogeneous photo-Fenton processes. Chemosphere 2021, 277, 130258. [Google Scholar] [CrossRef]
- Madejova, J.; Komadel, P. Baseline Studies Of The Clay Minerals Society Source Clays: Infrared Methods. Clays Clay Miner. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Figgis, B.N.; Lewis, J. The Magnetochemistry of Complex Compounds. In Modern Coordination Chemistry; Lewis, J., Wilkins, R.G., Eds.; Wiley: New York, NY, USA, 1960. [Google Scholar]
- Krupskaya, V.; Novikova, L.; Tyupina, E.; Belousov, P.; Dorzhieva, O.; Zakusin, S.; Kim, K.; Roessner, F.; Badetti, E.; Brunelli, A.; et al. The influence of acid modification on the structure of montmorillonites and surface properties of bentonites. Appl. Clay Sci. 2019, 172, 1–10. [Google Scholar] [CrossRef]
- Donohue, M.D.; Aranovich, G.L. Classification of Gibbs adsorption isotherms. Adv. Colloid Interface Sci. 1998, 76–77, 137–152. [Google Scholar] [CrossRef]
- Horikawa, T.; Do, D.D.; Nicholson, D. Capillary condensation of adsorbates in porous materials. Adv. Colloid Interface Sci. 2011, 169, 40–58. [Google Scholar] [CrossRef] [PubMed]
- Asaad, A.; Hubert, F.; Ferrage, E.; Dabat, T.; Paineau, E.; Porion, P.; Savoye, S.; Gregoire, B.; Dazas, B.; Delville, A.; et al. Role of interlayer porosity and particle organization in the diffusion of water in swelling clays. Appl. Clay Sci. 2021, 207, 106089. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, H.; Chai, P.; Bian, J.; Wang, X.; Liu, Y.; Yin, X.; Pan, S.; Pan, Z.H. Pore Characterization of Different Clay Minerals and Its Impact on Methane Adsorption Capacity. Energy Fuels. 2020, 34, 12204–12214. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Y.; Liu, J.; Liu, F.; Yu, Y. Experimental and theoretical studies on formaldehyde catalytic combustion over Cu–Fe spinel-type catalyst. Proc. Combust. Inst. 2021, 38, 6483–6491. [Google Scholar] [CrossRef]
- Tatarchuk, T.; Myslin, M.; Mironyuk, I.; Bououdina, M.; Pedziwiatr, A.T.; Gargula, R.; Bogacz, B.F.; Kurzydło, P. Synthesis, morphology, crystallite size and adsorption properties of nanostructured Mg–Zn ferrites with enhanced porous structure. J. Alloys Compd. 2020, 819, 152945. [Google Scholar] [CrossRef]
- Bellat, J.P.; Bezverkhyy, I.; Weber, G.; Royer, S.; Averlant, R.; Giraudon, J.M.; Lamonier, J.F. Capture of formaldehyde by adsorption on nanoporous materials. J. Hazard. Mater. 2015, 300, 711–717. [Google Scholar] [CrossRef]
- Detcheberry, M.; Destrac, P.; Meyer, X.M.; Condoret, J.-S. Phase equilibria of aqueous solutions of formaldehyde and metha-nol: Improved approach using UNI-QUAC coupled to chemical equilibria. Fluid Phase Equilib. 2015, 392, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Novikova, L.A.; Bogdanov, D.S.; Belchinskaya, L.I.; Kolousek, D.; Doushova, B.; Lhotka, M.; Petukhova, G.A. Adsorption of Formaldehyde from Aqueous Solutions Using Metakaolin-Based Geopolymer Sorbents. Prot. Met. Phys. Chem. Surf. 2019, 55, 864–871. [Google Scholar] [CrossRef]
- Bel’chinskaya, L.I.; Khodosova, N.A.; Strel’nikova, O.Y.; Petukhova, G.A.; Ciganda, L. Regulation of Sorption Processes on Natural Nanoporous Aluminosilicates. 1. Acidic and Basic Modifications. Prot. Met. Phys. Chem. Surf. 2015, 51, 779–786. [Google Scholar] [CrossRef]
- Kurian, M.; Thankachan, S.; Nair, D.S.; Aswathy, E.K.; Aswathy, B.; Arathy, T.; Binu Krishna, K.T. Structural, magnetic, and acidic properties of cobalt ferrite nanoparticles synthesised by wet chemical methods. J. Adv. Ceram. 2015, 4, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Fedotova, J.A.; Baev, V.G.; Lesnikovich, A.I.; Milevich, A.I.; Vorobjova, S.A. Magnetic properties and local configurations of 57Fe atoms in CoFe2O4 powders and CoFe2O4/PVA nanocomposites. Phys. Solid Stat. 2011, 53, 694–700. [Google Scholar] [CrossRef]







| Sample | Oxide Content, Mass % | LOI | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | MgO | K2O | CaO | TiO2 | Fe2O3 | Na2O | CoO | |||
| Bt | 67.67 | 12.55 | 1.22 | 1.04 | 2.01 | 4.21 | 10.33 | 0.10 | n.d. | 6.57 | 99.80 |
| Bt-10F | 53.90 | 11.85 | 1.35 | 0.83 | 1.48 | 3.05 | 18.04 | 0.11 | 4.70 | 4.52 | 99.92 |
| Bt-20F | 44.94 | 9.27 | 1.21 | 0.66 | 1.56 | 2.74 | 24.49 | 0.17 | 8.33 | 6.45 | 99.91 |
| Sample | SBET, m2/g | Pore Volume, cm3/g | Pore Diameter, D, nm | |||
|---|---|---|---|---|---|---|
| Vmicro | Vmeso | Vmacro * 50–75 nm | Vtotal | |||
| Bt | 85 | 0.0368 | 5.4862 | 2.1076 | 7.6306 | 7.0 |
| Bt-10F | 60 | 0 | 4.6870 | 2.2472 | 6.9342 | 4.9 |
| Bt-20F | 54 | 0 | 4.4298 | 2.2176 | 6.6474 | 6.6 |
| F | 16 | 0 | 1.7951 | 1.2553 | 3.0504 | 21.4 |
| Sample | Concentration of Equilibrium Solution | |||
|---|---|---|---|---|
| 0.038 M | 0.102 M | 0.201 M | 0.388 M | |
| Bt | 3.15 | 14.2 | 20.8 | 27.0 |
| Bt-10F | 4.0 | 14.8 | 21.5 | 28.6 |
| Bt-20F | 4.1 | 15.6 | 22.6 | 30.0 |
| F | 4.2 | 10.5 | 11.2 | 13.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khodosova, N.; Novikova, L.; Tomina, E.; Belchinskaya, L.; Zhabin, A.; Kurkin, N.; Krupskaya, V.; Zakusina, O.; Koroleva, T.; Tyupina, E.; et al. Magnetic Nanosorbents Based on Bentonite and CoFe2O4 Spinel. Minerals 2022, 12, 1474. https://doi.org/10.3390/min12111474
Khodosova N, Novikova L, Tomina E, Belchinskaya L, Zhabin A, Kurkin N, Krupskaya V, Zakusina O, Koroleva T, Tyupina E, et al. Magnetic Nanosorbents Based on Bentonite and CoFe2O4 Spinel. Minerals. 2022; 12(11):1474. https://doi.org/10.3390/min12111474
Chicago/Turabian StyleKhodosova, Nataliya, Lyudmila Novikova, Elena Tomina, Larisa Belchinskaya, Alexander Zhabin, Nikolay Kurkin, Victoria Krupskaya, Olga Zakusina, Tatiana Koroleva, Ekaterina Tyupina, and et al. 2022. "Magnetic Nanosorbents Based on Bentonite and CoFe2O4 Spinel" Minerals 12, no. 11: 1474. https://doi.org/10.3390/min12111474