As we all know, lead and zinc play an important role in people’s lives and are widely used in lead–acid batteries, rolled and extruded products, galvanization, radio-active shielding, alloy, pigments, brass and bronze, and chemicals for zinc [
1,
2,
3]. Today, they are mainly obtained from lead–zinc sulphide ores [
4]. Demand for lead and zinc is growing every year, and the total supply of lead and zinc is expected to reach the maximum of 13 Mt in 2030–2050 and 34 Mt in 2025–2030, respectively [
5]. In recent years, no new lead–zinc deposits have been found, and the depletion trend of lead–zinc resources has begun to emerge. With the gradual reduction of easy-to-dress ore, complex multi-metal difficult-to-dress ore will be a major problem in the future [
6,
7,
8]. Lead and zinc exist in nature mostly as sulphide and are often associated with other minerals, and they are often selected by flotation [
9]. However, due to the finer grain size of the lead–zinc minerals embedded in the gangue, the grain size of the gangue is very coarse, most of the energy in grinding is used to reduce the grain size of the gangue, and a lot of energy is wasted. On the other hand, grinding is a very energy-intensive process, which occupies 75% of the energy in the mineral processing process. At the same time, the energy utilization efficiency is very low, most of the energy is consumed by the machine itself, and only a small part of the energy is used for ore comminution [
10]. Lead-zinc ore contains carbon at the same time. Due to the good floatability of carbon, the grade of the concentrate will decrease, and the dosage of reagents will increase at the same time. Pan et al. [
11] explored the influence mechanism of carbon-containing materials in the flotation separation of lead–zinc ore. Through closed-circuit flotation tests, it was found that amorphous carbon accounted for a very high proportion of the total carbon, accounting for 99.7% of the total carbon in the concentrate, and had rich aromatic rings, carbonyl groups, and alkyl groups. In addition, the pore structure of amorphous carbon is rich, the specific surface area is 16.18 m
2/g, and the average pore size is 12.94 nm. After carbon pre-flotation, the grade and recovery of lead increased by 50.99% and 47.95%, respectively. Godirilwe et al. [
12] found that the presence of organic or inorganic carbon in the ore during flotation can have a negative impact on the product index, and they recovered a carbonaceous sulphide ore of 2.08 wt% copper, recovering only about 60% of the copper. Therefore, it is necessary to remove the carbon before flotation. Many studies have been carried out on the flotation of lead–zinc ore. Nowadays, the following methods are mainly used to effect the separation of lead–zinc sulfide: Wei [
13] used the new reagent to sort Pb–Zn minerals from low-grade complex sulfide ores, new reagent YZN as a zinc depressant, and new reagent BPB as a lead collector; the products had high recoveries and grades, and they also found that Na
2S could precipitate Pb
2+ and have a sulfide effect on oxidized Pb
2+. Oyelola et al. [
14] used gravity separation and froth flotation to separate low-grade zinc ore. Sahu et al. [
15] planed the comminution process according to the mineralogical characteristics of ore to avoid over-griding. Onal et al. [
16] added a gravity separation method before flotation, and used a gravity cyclone and rocker to pre-enrich the coarse particle size of the ore in advance. After that, the residual lead in the tailings was selected by flotation, and a high recovery rate of lead was obtained. In order to reveal the hydrothermal sulfidation mechanism of sulfur-bearing cerussite, Zheng et al. [
17] explored the interfacial exchange process between sulfur and carbonate ion disproportionation products by means of X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and Electron Probe Micro-Analysis-Energy Dispersive Spectroscopy (EPMA-EDS), providing guidance for the recovery of lead and other non-ferrous metals. He et al. [
18] studied the effect of hydration on the adsorption mechanism of benzohydroxamic acid (BHA) on the surface of cassiterite pre-adsorbed by lead ions (Pb
2+). When there is no lead ion (Pb
2+) adsorption on the surface of cassiterite, water molecules can be combined with the surface, and BHA cannot be adsorbed on the surface of the cassiterite; when the cassiterite surface is activated, BHA has a very low energy barrier and a very negative reaction energy difference with the lead ion (Pb
2+) on the cassiterite surface, indicating that BHA can be adsorbed on the cassiterite surface. Lan et al. [
19] studied the flotation performance of magnesite in the absence and presence of sulfur on the surface. Through XRD, XPS, and EPMA analysis, after thermochemical modification of magnesite in the presence of sulfur, ZnS and ZnS
2 were produced on the surface of zinc ore, and the flotation recovery of magnesium ore increased by about 65%. Kursun [
20] investigated the effects of the flotation column number and ultrasonic pretreatment on zinc recovery in three groups: firstly, the first group was subjected to only using single-stage column flotation without ultrasound, and the recovery rate of zinc was 29.41%; the second group differed from the first in that the ore was ultrasonically pretreated to obtain a recovery rate of 39.97% zinc; the third group used three stages of cleaning and three stages of scavenging flotation by column; and the final zinc recovery was 76.44%. Increased recovery means an increased contact angle and adhesion between the ore and the bubbles during flotation, which can be attributed to the dispersion of ultrasonic waves, increased collectors, and cavitation of bubbles. After a complex flotation process, the lead–zinc recovery index can reach about 80%–90%, but it is not suitable for large-scale industrial production because of its excessive number of reagents and complex processes. Bioleaching has been used to treat Pb–Zn ores, and although it has the advantage of high extraction rates, the leaching period is up to 24 d [
21]. In order to extract useful elements from low-grade lead–zinc ore, Lan et al. [
22] proposed a new roasting–dressing–leaching process. Although the recovery rates of zinc and lead are 86.04% and 69.08%, respectively, roasting will produce pollutants and consume fuel, which is not suitable for future environmental and resource policies. Enrichment of lead and zinc ores remains a challenge today, and many separation mechanisms have not been proposed. Information on the composition, embedding characteristics, crystal size, and crystal structure of minerals from a microscopic perspective is needed to reveal the flotation characteristics of different minerals during flotation. New theories are also needed to break through the technical limitations of Pb–Zn ore concentration. In general, the separation and enrichment of lead–zinc sulfide ore still poses great challenges, and many separation mechanisms have not yet been revealed. From the perspective of flotation, in order to achieve the separation of the target mineral and gangue, it is important to study the composition of the ore from a microscopic point of view, the characteristics of the distribution, the crystal size, and the crystal structure. At the same time, to break through the difficult problem of sorting lead–zinc ore, new theoretical support is needed.
Due to the complex structure of lead–zinc sulfide ore and the distribution of fine-grained minerals, the beneficiation of lead–zinc sulfide ore is difficult. To achieve efficient separation, it is critical to design the separation process based on the characteristics of the ore. Micro-grain lead and zinc minerals coexist closely in the ore with the minerals. A suitable grinding fineness is not only related to the liberation of useful minerals, but also affects the separation efficiency of useful minerals in the flotation process [
23]. According to the ore’s mineralogical characteristics, researchers have made many explorations in enrichment and separation. Jian et al. [
24] found that the crush size clearly affected the heavy liquid separation process, and using a biconical dense medium cyclone (BDMC) to remove gangue from a low-grade Pb–Zn sulfide ore, it was finally determined that the gangue removal effect was the best when the particle size was in the range of −13 + 1 ~ −20 + 1 mm. After optimizing the BDMC operation process, the recovery rates of lead and zinc were 7.92% and 12.50%, respectively. In order to minimize the production and utilization of tailings, Souza [
25] analyzed the properties of the tailings through physical, chemical, and mineralogical characterization, focusing on quantitative electron microscopy (QEM), and found that its main phases were hematite and quartz, followed by goethite, bauxite trihydrate, and kaolinite, and obtained the degree of dissociation, which provided an important basis for the utilization of tailings. Yong et al. [
26] selected six typical common magnetic iron concentrates in China, studied the mineralogical characteristics of iron concentrates, revealed the intrinsic connection between the mineralogical characteristics of raw materials and sorting indexes, and established an evaluation system for the preparation of super iron concentrates based on the mineralogical characteristics of raw materials. However, the relationship between the mineralogical characteristics of Pb–Zn ores and roughing has not been developed, so it is necessary to establish a beneficiation method for Pb–Zn ores.
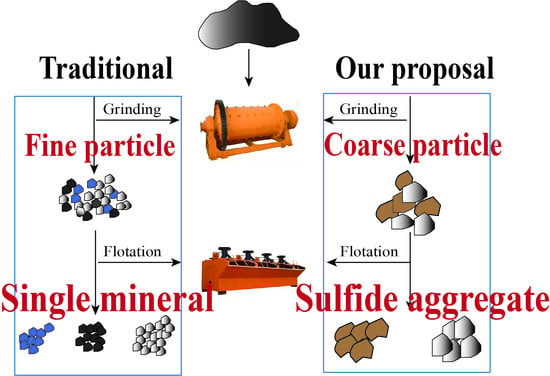
Lead-zinc ore is difficult to separate because of its polymetallic fractionation, fine dissemination, low grade, and low degree of liberation. Conventional lead–zinc ore beneficiation methods often liberate useful mineral monomers from the ore, a process that consumes a lot of energy; then, a series of corresponding flotation processes are designed, using a large variety of reagents and high dosages, which has the disadvantage that different lead–zinc sulfide ores require different types of reagents, and the effect is not significantly enhanced. Therefore, a Mineral Liberation Analyzer (MLA) is introduced in this paper to observe the degree of liberation of particles or aggregates after grinding and to select the appropriate particle size in order to reduce the energy consumption during the grinding process. For example, copper and zinc are finely distributed in the ore, but both are present in the mineral in the form of sulphide, so the sulphide aggregate can be considered as the target mineral for flotation. The mineralogical characteristics of ore and mineral aggregates after crushing and grinding are listed in this paper. The particle size distribution characteristics of aggregate and single minerals are explored, and the optimal process parameters of flotation after the rough grinding of lead–zinc ore are determined, which provides theoretical guidance for similar polymetallic sulfide ores.
The sulfide ore used in this paper comes from the Inner Mongolia Autonomous Region of China. The ore contains a high cryptocrystalline graphite, low lead and zinc content, and fine intercalation particle size, but the sulfide aggregate has a coarser particle size and simple intergrowth relationship. Therefore, the improvement in zinc recovery depends on the recovery of lead–zinc sulfide.