Phosphorus Species in Deep-Sea Carbonate Deposits: Implications for Phosphorus Cycling in Cold Seep Environments
Abstract
:1. Introduction
2. Geological Setting and Sampling
3. Methods
3.1. Light Microscopy
3.2. X-ray Diffraction (XRD)
3.3. Major and Trace Element Analyses
3.4. Sequential P Extraction
3.5. Raman Spectroscopy and Imagery
3.6. Scanning Electron Microscopy and Element Mapping
3.7. NanoSIMS Ion Mapping
4. Results
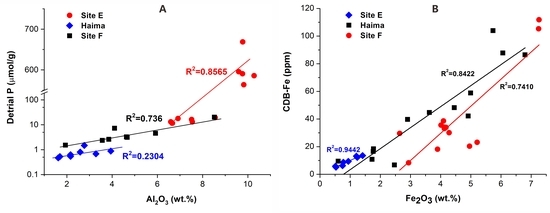
4.1. Mineralogy and Geochemistry of the Carbonates
4.2. P species Determined by Sequential P Extraction
4.3. Identification of Organic-P in the Carbonates
4.4. Identification of Detrital-P and Authigenic Ca-P in the Carbonates
5. Discussion
5.1. Distribution of P Species at Cold Seeps
5.2. Origin and Cycling of P at Cold Seeps
5.3. A Potential Model for P Cycling in Cold Seep Environments
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Egger, M.; Jilbert, T.; Behrends, T.; Rivard, C.; Slomp, C.P. Vivianite is a major sink for phosphorus in methanogenic coastal surface sediments. Geochim. Cosmochim. Acta 2015, 169, 217–235. [Google Scholar] [CrossRef] [Green Version]
- Kraal, P.; Dijkstra, N.; Behrends, T.; Slomp, C.P. Phosphorus burial in sediments of the sulfidic deep Black Sea: Key roles for adsorption by calcium carbonate and apatite authigenesis. Geochim. Cosmochim. Acta 2017, 204, 140–158. [Google Scholar] [CrossRef]
- Feng, D.; Qiu, J.W.; Hu, Y.; Peckmann, J.; Guan, H.X.; Tong, H.P.; Chen, C.; Chen, J.X.; Gong, S.G.; Li, N.; et al. Cold seep systems in the South China Sea: An overview. J. Asian Earth Sci. 2018, 168, 3–16. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Milucka, J.; Ferdelman, T.G.; Polerecky, L.; Franzke, D.; Wegener, G.; Schmid, M.; Lieberwirth, I.; Wagner, M.; Widdel, F.; Kuypers, M.M. Zero-valent sulphur is a key intermediate in marine methane oxidation. Nature 2012, 491, 541–546. [Google Scholar] [CrossRef]
- Diaz-del-Rio, V.; Somoza, L.; Martinez-Frias, J.; Mata, M.P.; Delgado, A.; Hernandez-Molina, F.J.; Lunar, R.; Martin-Rubi, J.A.; Maestro, A.; Fernandez-Puga, M.C.; et al. Vast fields of hydrocarbon-derived carbonate chimneys related to the accretionary wedge/olistostrome of the Gulf of Cádiz. Mar. Geol. 2003, 195, 177–200. [Google Scholar] [CrossRef]
- Pierre, C.; Fouquet, Y. Authigenic carbonates from methane seeps of the Congo deep-sea fan. Geo-Mar. Lett. 2007, 27, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Pierre, C.; Bayon, G.; Blanc-Valleron, M.M.; Mascle, J.; Dupré, S. Authigenic carbonates related to active seepage of methane-rich hot brines at the Cheops mud volcano, Menes caldera (Nile deep-sea fan, eastern Mediterranean Sea). Geo-Mar. Lett. 2014, 34, 253–267. [Google Scholar] [CrossRef] [Green Version]
- Ruffine, L.; Caprais, J.-C.; Bayon, G.; Riboulot, V.; Donval, J.-P.; Etoubleau, J.; Birot, D.; Pignet, P.; Rongemaille, E.; Chazallon, B.; et al. Investigation on the geochemical dynamics of a hydrate-bearing pockmark in the Niger Delta. Mar. Pet. Geol. 2013, 43, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Viola, I.; Oppo, D.; Franchi, F.; Capozzi, R.; Dinelli, E.; Liverani, B.; Taviani, M. Mineralogy, geochemistry and petrography of methane-derived authigenic carbonates from Enza River, Northern Apennines (Italy). Mar. Pet. Geol. 2015, 66, 566–581. [Google Scholar] [CrossRef]
- Viola, I.; Capozzi, R.; Bernasconi, S.M.; Rickli, J. Carbon, oxygen and strontium isotopic constraints on fluid sources, temperatures and biogeochemical processes during the formation of seep carbonates-Secchia River site, Northern Apennines. Sediment. Geol. 2017, 357, 1–15. [Google Scholar] [CrossRef]
- Peng, X.; Guo, Z.; Chen, S.; Sun, Z.; Xu, H.; Ta, K.; Zhang, J.; Zhang, L.; Li, J.; Du, M. Formation of carbonate pipes in the northern Okinawa Trough linked to strong sulfate exhaustion and iron supply. Geochim. Cosmochim. Acta 2017, 205, 1–13. [Google Scholar] [CrossRef]
- Egger, M.; Hagens, M.; Sapart, C.J.; Dijkstra, N.; Van Helmond, N.A.G.M.; Mogollón, J.M.; Risgaard-Petersen, N.; Van Der Veen, C.; Kasten, S.; Riedinger, N.; et al. Iron oxide reduction in methane-rich deep Baltic Sea sediments. Geochim. Cosmochim. Acta 2017, 207, 256–276. [Google Scholar] [CrossRef]
- Feng, D.; Chen, D.F. Authigenic carbonates from an active cold seep of the northern South China Sea: New insights into fluid sources and past seepage activity. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 122, 74–83. [Google Scholar] [CrossRef]
- Lin, Z.; Sun, X.; Peckmann, J.; Lu, Y.; Xu, L.; Strauss, H.; Zhou, H.; Gong, J.; Lu, H.; Teichert, B.M.A. How sulfate-driven anaerobic oxidation of methane affects the sulfur isotopic composition of pyrite: A SIMS study from the South China Sea. Chem. Geol. 2016, 440, 26–41. [Google Scholar] [CrossRef]
- Peckmann, J.; Reimer, A.; Luth, U.; Luth, C.; Hansen, B.T.; Heinicke, C.; Hoefs, J.; Reitner, J. Methane-derived carbonates and authigenic pyrite from the northwestern Black Sea. Mar. Geol. 2001, 177, 129–150. [Google Scholar] [CrossRef]
- Peckmann, J.; Birgel, D.; Kiel, S. Molecular fossils reveal fluid composition and flow intensity at a Cretaceous seep. Geology 2009, 37, 847–850. [Google Scholar] [CrossRef]
- Peckmann, J.; Thiel, V. Carbon cycling at ancient methane–seeps. Chem. Geol. 2004, 205, 443–467. [Google Scholar] [CrossRef]
- Feng, D.; Peng, Y.; Bao, H.; Peckmann, J.; Roberts, H.H.; Chen, D. A carbonate-based proxy for sulfate-driven anaerobic oxidation of methane. Geology 2016, 44, 999–1002. [Google Scholar] [CrossRef] [Green Version]
- Liang, Q.; Hu, Y.; Feng, D.; Peckmann, J.; Chen, L.; Yang, S.; Liang, J.; Tao, J.; Chen, D. Authigenic carbonates from newly discovered active cold seeps on the northwestern slope of the South China Sea: Constraints on fluid sources, formation environments, and seepage dynamics. Deep Sea Res. Part I Oceanogr. Res. Pap. 2017, 124, 31–41. [Google Scholar] [CrossRef]
- Liu, C.S.; Morita, S.; Liao, Y.H.; Ku, C.K.; Machiyama, H.; Lin, S.; Soh, W. High-resolution seismic images of the formosa ridge off southwestern taiwan where “hydrothermal” chemosynthetic community is present at a cold seep site. In Proceedings of the 6th International Conference on Gas Hydrates, Vancouver, BC, Canada, 6–10 July 2008. [Google Scholar]
- Li, J.; Peng, X.; Bai, S.; Chen, Z.; Van Nostrand, J.D. Biogeochemical processes controlling authigenic carbonate formation within the sediment column from the Okinawa Trough. Geochim. Cosmochim. Acta 2018, 222, 363–382. [Google Scholar] [CrossRef]
- Sun, Z.; Wei, H.; Zhang, X.; Shang, L.; Yin, X.; Sun, Y.; Xu, L.; Huang, W.; Zhang, X. A unique Fe-rich carbonate chimney associated with cold seeps in the Northern Okinawa Trough, East China Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2015, 95, 37–53. [Google Scholar] [CrossRef]
- Sibuet, M.; Olu, K. Biogeography, biodiversity and fluid dependence of deep-sea cold-seep communities at active and passive margins. Deep Sea Res. Part II Top. Stud. Oceanogr. 1998, 45, 517–567. [Google Scholar] [CrossRef]
- Wu, Z.; Li, J.; Jin, X.; Shang, J.; Li, S.; Jin, X. Distribution, features, and influence factors of the submarine topographic boundaries of the Okinawa Trough. Sci. China Earth Sci. 2014, 57, 1885–1896. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. Development of a sequential extraction method for different forms of phosphorus in marine-sediments. Limnol. Oceanogr. 1992, 37, 1460–1482. [Google Scholar] [CrossRef]
- Slomp, C.P.; Epping, E.H.G.; Helder, W.; Van Raaphorst, W. A key role for iron-bound phosphorus in authigenic apatite formation in North Atlantic continental platform sediments. J. Mar. Res. 1996, 54, 1179–1205. [Google Scholar] [CrossRef]
- Kraal, P.; Slomp, C.P.; Forster, A.; Kuypers, M.M.M.; Sluijs, A. Pyrite oxidation during sample storage determines phosphorus fractionation in carbonate-poor anoxic sediments. Geochim. Cosmochim. Acta 2009, 73, 3277–3290. [Google Scholar] [CrossRef] [Green Version]
- Murphy, J.; Riley, J.P. A Single-Solution Method for the Determination of Soluble Phosphate in Sea Water. J. Mar. Biol. Assoc. UK 1958, 37, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Ammar, M.R.; Rouzaud, J.N. How to obtain a reliable structural characterization of polished graphitized carbons by Raman microspectroscopy. J. Raman Spectrosc. 2012, 43, 207–211. [Google Scholar] [CrossRef]
- Peng, X.; Guo, Z.; House, C.H.; Chen, S.; Ta, K. SIMS and NanoSIMS analyses of well-preserved microfossils imply oxygen-producing photosynthesis in the Mesoproterozoic anoxic ocean. Chem. Geol. 2016, 441, 24–34. [Google Scholar] [CrossRef]
- Katayama, H.; Watanabe, Y. The Huanghe and Changjiang contribution to seasonal variability in terrigenous particulate load to the Okinawa Trough. Deep Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 475–485. [Google Scholar] [CrossRef]
- Sibuet, J.C.; Letouzey, J.; Barbier, F.; Charvet, J.; Foucher, J.-P.; Hilde, T.W.C.; Kimura, M.; Chiao, L.-Y.; Marsset, B.; Muller, C.; et al. Back Arc Extension in the Okinawa Trough. J. Geophys. Res. Sol. Ea. 1987, 92, 14041–14063. [Google Scholar] [CrossRef] [Green Version]
- Ruttenberg, K.C. The global phosphorus cycle. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 10, pp. 499–558. [Google Scholar]
- Ruttenberg, K.C. The global phosphorus cycle. In Treatise on Geochemistry; Turekian, K.K., Holland, D.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 585–643. [Google Scholar]
- Bayon, G.; Dupré, S.; Ponzevera, E.; Etoubleau, J.; Chéron, S.; Pierre, C.; Mascle, J.; Boetius, A.; De Lange, G.J. Formation of carbonate chimneys in the Mediterranean Sea linked to deep-water oxygen depletion. Nat. Geosci. 2013, 6, 755. [Google Scholar] [CrossRef]
- De Kanel, J.; Morse, J.W. The chemistry of orthophosphate uptake from seawater on to calcite and aragonite. Geochim. Cosmochim. Acta 1978, 42, 1335–1340. [Google Scholar] [CrossRef]
- März, C.; Riedinger, N.; Sena, C.; Kasten, S. Phosphorus dynamics around the sulphate-methane transition in continental margin sediments: Authigenic apatite and Fe(II) phosphates. Mar. Geol. 2018, 404, 84–96. [Google Scholar] [CrossRef] [Green Version]
- Stumm, W.; Leckie, J.O. Phosphate exchange with sediments: Its role in the productivity of surface waters. Advan. Water Pollut. Res. 1971, 5, 1–26. [Google Scholar]
- Millero, F.; Huang, F.; Zhu, X.; Liu, X.; Zhang, J.Z. Adsorption and Desorption of Phosphate on Calcite and Aragonite in Seawater. Aquat. Geochem. 2001, 7, 33–56. [Google Scholar] [CrossRef]
- März, C.; Poulton, S.W.; Wagner, T.; Schnetger, B.; Brumsack, H.J. Phosphorus burial and diagenesis in the central Bering Sea (Bowers Ridge, IODP Site U1341): Perspectives on the marine P cycle. Chem. Geol. 2014, 363, 270–282. [Google Scholar] [CrossRef]
- Dijkstra, N.; Kraal, P.; Kuypers, M.M.; Schnetger, B.; Slomp, C.P. Are iron-phosphate minerals a sink for phosphorus in anoxic Black Sea sediments? PLoS ONE 2014, 9, e101139. [Google Scholar] [CrossRef]
- Nembrini, G.P.; Capobianco, J.A.; Viel, M.; Williams, A.F. A Mössbauer and chemical study of the formation of vivianite in sediments of Lago Maggiore (Italy). Geochim. Cosmochim. Acta 1983, 47, 1459–1464. [Google Scholar] [CrossRef]
- Slomp, C.P.; Mort, H.P.; Jilbert, T.; Reed, D.C.; Gustafsson, B.G.; Wolthers, M. Coupled dynamics of iron and phosphorus in sediments of an oligotrophic coastal basin and the impact of anaerobic oxidation of methane. PLoS ONE 2013, 8, e62386. [Google Scholar] [CrossRef] [PubMed]
- Dellwig, O.; Leipe, T.; März, C.; Glockzin, M.; Pollehne, F.; Schnetger, B.; Yakushev, E.V.; Böttcher, M.E.; Brumsack, H.-J. A new particulate Mn–Fe–P-shuttle at the redoxcline of anoxic basins. Geochim. Cosmochim. Acta 2010, 74, 7100–7115. [Google Scholar] [CrossRef]
- Berner, R.A.; Rao, J.L. Phosphorus in sediments of the Amazon River and estuary: Implications for the global flux of phosphorus to the sea. Geochim. Cosmochim. Acta 1994, 58, 2333–2339. [Google Scholar] [CrossRef]
- Egger, M.; Kraal, P.; Jilbert, T.; Sulu-Gambari, F.; Sapart, C.J.; Röckmann, T.; Slomp, C.P. Anaerobic oxidation of methane alters sediment records of sulfur, iron and phosphorus in the Black Sea. Biogeosciences 2016, 13, 5333–5355. [Google Scholar] [CrossRef] [Green Version]
- Jilbert, T.; Slomp, C.P. Iron and manganese shuttles control the formation of authigenic phosphorus minerals in the euxinic basins of the Baltic Sea. Geochim. Cosmochim. Acta 2013, 107, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Kraal, P.; Burton, E.D.; Rose, A.L.; Kocar, B.D.; Lockhart, R.S.; Grice, K.; Bush, R.T.; Tan, E.; Webb, S.M. Sedimentary iron–phosphorus cycling under contrasting redox conditions in a eutrophic estuary. Chem. Geol. 2015, 392, 19–31. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl. Environ. Microbiol. 1987, 53, 2636–2641. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.M.; Beard, B.L.; Roden, E.E. The Iron Isotope Fingerprints of Redox and Biogeochemical Cycling in Modern and Ancient Earth. Annu. Rev. Earth Pl. Sci. 2008, 36, 457–493. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Izon, G.; Wang, J.; Antler, G.; Wang, Z.; Zhao, J.; Egger, M. Vivianite formation in methane-rich deep-sea sediments from the South China Sea. Biogeosciences 2018, 15, 6329–6348. [Google Scholar] [CrossRef] [Green Version]
- Riedinger, N.; Formolo, M.J.; Lyons, T.W.; Henkel, S.; Beck, A.; Kasten, S. An inorganic geochemical argument for coupled anaerobic oxidation of methane and iron reduction in marine sediments. Geobiology 2014, 12, 172–181. [Google Scholar] [CrossRef]
- Sivan, O.; Adler, M.; Pearson, A.; Gelman, F.; Bar-Or, I.; John, S.G.; Eckert, W. Geochemical evidence for iron-mediated anaerobic oxidation of methane. Limnol. Oceanogr. 2011, 56, 1536–1544. [Google Scholar] [CrossRef] [Green Version]
- Segarra, K.E.A.; Comerford, C.; Slaughter, J.; Joye, S.B. Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim. Cosmochim. Acta 2013, 115, 15–30. [Google Scholar] [CrossRef]
- Sivan, O.; Antler, G.; Turchyn, A.V.; Marlow, J.J.; Orphan, V.J. Iron oxides stimulate sulfate-driven anaerobic methane oxidation in seeps. Proc. Natl. Acad. Sci. USA 2014, 111, E4139–E4147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beal, E.J.; House, C.H.; Orphan, V.J. Manganese- and iron-dependent marine methane oxidation. Science 2009, 325, 184–187. [Google Scholar] [CrossRef] [Green Version]
- Holmkvist, L.; Ferdelman, T.G.; Jorgensen, B.B. A cryptic sulfur cycle driven by iron in the methane zone of marine sediment (Aarhus Bay, Denmark). Geochim. Cosmochim. Acta 2011, 75, 3581–3599. [Google Scholar] [CrossRef]
- Lichtschlag, A.; Kamyshny, A.; Ferdelman, T.G.; DeBeer, D. Intermediate sulfur oxidation state compounds in the euxinic surface sediments of the Dvurechenskii mud volcano (Black Sea). Geochim. Cosmochim. Acta 2013, 105, 130–145. [Google Scholar] [CrossRef]
- Filippelli, G.M. The global phosphorus cycle: Past, present, and future. Elements 2008, 4, 89–95. [Google Scholar] [CrossRef]
- Ruttenberg, K.C.; Berner, R.A. Authigenic apatite formation and burial in sediments from non-upwelling, continental-margin environments. Geochim. Cosmochim. Acta 1993, 57, 991–1007. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, N.; Cao, H.; Xu, C.; Liu, L.; Yin, X.; Zhang, X.R.; Geng, W.; Zhang, X.L. Hydrothermal metal supplies enhance the benthic methane filter in oceans: An example from the okinawa trough. Chem. Geol. 2019, 525, 190–209. [Google Scholar] [CrossRef]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Du, M.; Li, J.; Xu, H.; Ta, K.; Chen, S.; Peng, X. Phosphorus Species in Deep-Sea Carbonate Deposits: Implications for Phosphorus Cycling in Cold Seep Environments. Minerals 2020, 10, 645. https://doi.org/10.3390/min10070645
Zhou J, Du M, Li J, Xu H, Ta K, Chen S, Peng X. Phosphorus Species in Deep-Sea Carbonate Deposits: Implications for Phosphorus Cycling in Cold Seep Environments. Minerals. 2020; 10(7):645. https://doi.org/10.3390/min10070645
Chicago/Turabian StyleZhou, Junlie, Mengran Du, Jiwei Li, Hengchao Xu, Kaiwen Ta, Shun Chen, and Xiaotong Peng. 2020. "Phosphorus Species in Deep-Sea Carbonate Deposits: Implications for Phosphorus Cycling in Cold Seep Environments" Minerals 10, no. 7: 645. https://doi.org/10.3390/min10070645