Adsorption Behavior and Wettability of Rhodochrosite Surface: Effect of C18 Fatty Acid Unsaturation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Measurements of Surface Tension
2.2.2. Measurements of Contact Angle
2.2.3. AFM Analysis
3. Results and Discussion
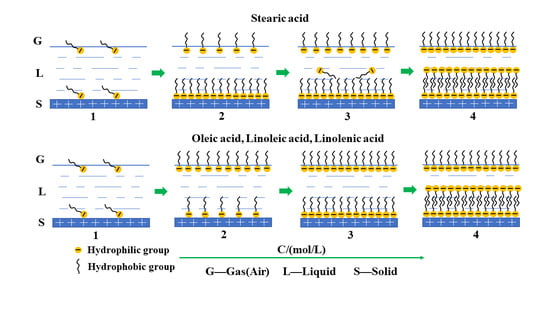
3.1. Self-Assembly of Fatty Acid in Aqueous Solution
3.2. Wettability of Rhodochrosite
3.3. Adsorption at Rhodochrosite-Water and Air-Water Interface
3.3.1. Dependence of Wettability and Surface Tension
3.3.2. Relationship between the Adhesion and Surface Tension
3.3.3. Work of Adhesion
3.4. Structural Dependence of Fatty Acid on the Wettability of Rhodochrosite Surface
3.5. Morphology of Adsorbed Fatty Acid on Rhodochrosite Surface
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chau, T.T.; Bruckard, W.J.; Koh, P.T.L.; Nguyen, A.V. A review of factors that affect contact angle and implications for flotation practice. Adv. Colloid Interface Sci. 2009, 150, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Effect of electrolytes on wettability of glass surface using anionic and cationic surfactant solutions. J. Colloid Interface Sci. 2014, 413, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, X.; Guo, L.; Xu, Z.; Zhang, L.; Gong, Q.; Zhang, L.; Zhao, S. Effect of adsorption of catanionic surfactant mixtures on wettability of quartz surface. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 564–573. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Butterworth-Heinemann Elsevier Ltd.: Oxford, UK, 2016. [Google Scholar]
- Wang, G.; Nguyen, A.V.; Mitra, S.; Joshi, J.B.; Jameson, G.J.; Evans, G.M. A review of the mechanisms and models of bubble-particle detachment in froth flotation. Sep. Purif. Technol. 2016, 170, 155–172. [Google Scholar] [CrossRef] [Green Version]
- Han, C.; Li, T.; Zhang, W.; Zhang, H.; Zhao, S.; Ao, Y.; Wei, D.; Shen, Y. Density Functional Theory Study on the Surface Properties and Floatability of Hemimorphite and Smithsonite. Minerals 2018, 8, 542. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, S.K.; Mallick, M.K.; Singh, V.; Murthy, Y.R. Preliminary studies on teeter bed separator for separation of manganese fines. Powder Technol. 2013, 239, 284–289. [Google Scholar] [CrossRef]
- Altun, N.E.; Hicyilmaz, C.; Hwang, J.Y.; Bagci, A.S. Evaluation of a Turkish low quality oil shale by flotation as a clean energy source: Material characterization and determination of flotation behavior. Fuel Process. Technol. 2006, 87, 783–791. [Google Scholar] [CrossRef]
- Rao, S.R. Surface Chemistry of Froth; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Fuerstenau, M.C.; Jameson, G.J.; Yoon, R.H. Froth Flotation: A Century of Innovation; Society for Mining, Metallurgy, and Exploration, Inc.: Englewood, CO, USA, 2007. [Google Scholar]
- Cao, X.; Lu, J.A.; Zhang, G. Flotation processing research of a low-grade manganese carbonate ore. Metal Mine 2013, 42, 99–101. [Google Scholar]
- Fuerstenau, D.W.; Shibata, J. On using electrokinetics to interpret the flotation and interfacial behavior of manganese dioxide. Int. J. Miner. Process. 1999, 57, 205–217. [Google Scholar] [CrossRef]
- Andrade, E.M.; Costa, B.L.C.M.; Alcantara, G.A.G.; Lima, R.M.F. Flotation of manganese minerals and quartz by sodium oleate and water glass. Lat. Am. Appl. Res. 2012, 42, 39–43. [Google Scholar]
- Teng, Q.; Feng, Y.L.; Li, H.R.; Yang, Z.C. Dodecylamine flotation separation of rhodochrosite from calcite and its mechanism. Chin. J. Nonferr. Metals 2014, 24, 2676–2683. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhong, H.; Tan, X.; Zhan, J. Research progress in cationic collectors. Conserv. Util. Miner. Resour. 2011, 44–49. [Google Scholar] [CrossRef]
- Song, S.; Lopez-Valdivieso, A.; Ding, Y. Effects of nonpolar oil on hydrophobic flocculation of hematite and rhodochrosite fines. Powder Technol. 1999, 101, 73–80. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Brandão, P.R.G.; Poling, G.W. Anionic flotation of magnesite. Can. Metall. Quart. 1982, 21, 211–220. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Q.; Li, X.; Wang, X.; Ke, B.; Li, X.; Shen, Z. Effect of the morphology of adsorbed oleate on the wettability of a collophane surface. Appl. Surf. Sci. 2018, 444, 87–96. [Google Scholar] [CrossRef]
- Xie, J.; Li, X.; Mao, S.; Li, L.; Ke, B.; Zhang, Q. Effects of structure of fatty acid collectors on the adsorption of fluorapatite (0 0 1) surface: A first-principles calculations. Appl. Surf. Sci. 2018, 444, 699–709. [Google Scholar] [CrossRef]
- Lu, Y.; Drelich, J.; Miller, J.D. Oleate adsorption at an apatite surface studied by ex-situ FTIR internal reflection spectroscopy. J. Colloid Interface Sci. 1998, 202, 462–476. [Google Scholar] [CrossRef]
- Young, C.A.; Miller, J.D. Effect of temperature on oleate adsorption at a calcite surface: An FT-NIR/IRS study and review. Int. J. Miner. Process. 2000, 58, 331–350. [Google Scholar] [CrossRef]
- Rao, K.H.; Forssberg, K.S.E. Mechanism of fatty acid adsorption in salt-type mineral flotation. Miner. Eng. 1991, 4, 879–890. [Google Scholar] [CrossRef]
- Iwasaki, I.; Cooke, S.R.B.; Choi, H.S. Flotation characteristics of hematite, goethite and acitivated quartz with 18-carbon aliphatic acids and related compounds. Trans. AIME 1960, 217, 237–244. [Google Scholar]
- Purcell, G.; Sun, S.C. Significance of double bonds in fatty acid flotation-an electrokinetic study—A flotation study. Trans. AIME 1963, 226, 13–16. [Google Scholar]
- Liu, R.; Zhang, G.; Zhang, H.; Liu, L.; He, L.; Chen, Y. Effect of iodine value of sodium fatty acids on flotation of collophanite. Physicochem. Probl. Miner. Process. 2019, 55, 770–778. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Zhang, Q.; Lu, D.; Hu, Y. Flotation separation of diaspore from aluminosilicates using commercial oleic acids of different iodine values. Int. J. Miner. Process. 2017, 168, 95–101. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Wang, H.; Sun, Q.; Wang, Q.; Alshameri, A. Flotation behavior of four C18 hydroxamic acids as collectors of rhodochrosite. Miner. Eng. 2015, 78, 15–20. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, T.; Yan, C.; Liang, H.; Chen, T.; Li, D.; Wang, Q. The flotation of low-grade manganese ore using a novel linoleate hydroxamic acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 1–9. [Google Scholar] [CrossRef]
- Chen, J.Q.; Zhou, J.; Zou, Y.K.; Wan, Y.Y.; Chen, H.; Zhou, C.H.; Chen, T.; Yan, C.J. Flotation behavior and mechanism of low-grade manganese ore using N-hydroxyethyl fatty acid amide. Chin J. Nonferr. Metals 2018, 28, 1059–1066. (In Chinese) [Google Scholar] [CrossRef]
- Dai, T.; Wang, S.; Zhong, H. Flotation performance and adsorption mechanism of alkyl-amide-hydroxime acid to rhodochrosite. China’ Manganese Ind. 2018, 36, 142–146. (In Chinese) [Google Scholar] [CrossRef]
- Qin, W.; Zou, S.; Liu, S.; Luo, H.; Liu, R.; Wang, X. Solution chemistry mechanism of flotation of sodium oleate on rhodochrosite. J. Wuhan Univ. Technol. 2014, 36, 124–129. [Google Scholar] [CrossRef]
- Bu, Y.; Liu, R.; Sun, W.; Hu, Y. Synergistic mechanism between SDBS and oleic acid in anionic flotation of rhodochrosite. Int. J. Miner. Metall. Mater. 2015, 22, 447–452. [Google Scholar] [CrossRef]
- Zhao, G.; Dai, T.; Wang, S.; Zhong, H. Study on a novel hydroxamic acid as the collector of rhodochrosite. Physicochem. Probl. Miner. Process. 2018, 54, 428–439. [Google Scholar] [CrossRef]
- Tomoaia-Cotişel, M.; Zsako, J.N.; Mocanu, A.; Lupea, M.; Chifu, E. Insoluble mixed monolayers: III. The ionization characteristics of some fatty acids at the air/water interface. J. Colloid Interface Sci. 1987, 117, 464–476. [Google Scholar] [CrossRef]
- Hifeda, Y.M.; Rayfield, G.W. Phase transitions in fatty acid monolayers containing a single double bond in the fatty acid tail. J. Colloid Interface Sci. 1985, 104, 209–215. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J. Colloid Interface Sci. 2002, 256, 201–207. [Google Scholar] [CrossRef]
- Davies, J.T.; Rideal, E.K. Interfacial Phenomena; Academic Press: New York, NY, USA, 1961. [Google Scholar]
- Peltonen, J.P.K.; Rosenholm, J.B. Thin Solid Films. The influence of light on the properties of fatty acid-poly(3-octylthiophene) Langmuir-Blodgett films. Thin Solid Films 1989, 179, 543–547. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q. Insight into the Influence of Surface Roughness on the Wettability of Apatite and Dolomite. Minerals 2020, 10, 114. [Google Scholar] [CrossRef] [Green Version]
- Xie, J.; Zhang, Q.; Mao, S.; Li, X.; Shen, Z.; Li, L. Anisotropic crystal plane nature and wettability of fluorapatite. Appl. Surf. Sci. 2019, 493, 294–307. [Google Scholar] [CrossRef]
- Jin, G. Surfactant Chemistry, 2nd ed.; University of Science and Technology of China Press: Hefei, China, 2013. [Google Scholar]
- Jiang, Y.C.; Ye, J.P.; Wu, S.K. Premicelle formation in surfactant solution and measurement of its average aggregation number. Acta Chim. Sin. 1992, 50, 1080–1084. (In Chinese) [Google Scholar]
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Bai, S.; Liu, D. A mixed collector system for phosphate flotation. Miner. Eng. 2015, 78, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Jiang, L.; Shen, Q.; Shen, J.; Hana, Y.; Hongman, Z. Investigation on the self-assembled behaviors of C18 unsaturated fatty acids in arginine aqueous solution. RSC Adv. 2017, 7, 41561–41572. [Google Scholar] [CrossRef] [Green Version]
- Lundgren, S.M.; Persson, K.; Mueller, G.; Kronberg, B.; Clarke, J.; Chtaib, M.; Claesson, P.M. Unsaturated fatty acids in alkane solution: Adsorption to steel surfaces. Langmuir 2007, 23, 10598–10602. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.H.; Casford, M.T.; Steitz, R.; Zarbakhsh, A.; Welbourn, R.J.L.; Clarke, S.M. Comparative adsorption of saturated and unsaturated fatty acids at the iron oxide/oil interface. Langmuir 2016, 32, 534–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernett, M.K.; Zisman, W.A. Relation of wettability by aqueous solutions to the surface constitution of low-energy solids. J. Phys. Chem. 1959, 63, 1241–1246. [Google Scholar] [CrossRef]
- Bernett, M.K.; Zisman, W.A. Wetting of low-energy solids by aqueous solutions of highly fluorinated acids and salts. J. Phys. Chem. 1959, 63, 1911–1916. [Google Scholar] [CrossRef]
- Szymczyk, K.; Zdziennicka, A.; Jańczuk, B.; Wójcik, W. The wettability of polytetrafluoroethylene and polymethyl methacrylate by aqueous solution of two cationic surfactants mixture. J. Colloid Interface Sci. 2006, 293, 172–180. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Janczuk, B.; Wójcik, W. Wettability of polytetrafluoroethylene by aqueous solutions of two anionic surfactant mixtures. J. Colloid Interface Sci. 2003, 268, 200–207. [Google Scholar] [CrossRef]
- Bargeman, D.; Van Voorst Vader, F. Effect of surfactants on contact angles at nonpolar solids. J. Colloid Interface Sci. 1973, 42, 467–472. [Google Scholar] [CrossRef]
- Lucassen-Reynders, E.H. Surface equation of state for ionized surfactants. J. Phys. Chem. 1966, 70, 1777–1785. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Li, Z.; Zhang, L.; Xu, Z.; Zhao, S.; Yu, J. Wettability of a quartz surface in the presence of four cationic surfactants. Langmuir 2010, 26, 18834–18840. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Xu, Z.; Liu, D.; Song, X.; Cao, X.; Zhang, L.; Zhao, S. Effect of zwitterionic surfactants on wetting of quartz surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2013, 430, 110–116. [Google Scholar] [CrossRef]
- Zisman, W.A. Contact angle, wettability and adhesion. In Advances in Chemistry Series; Gould, R.F., Ed.; American Chemical Society: Washington, DC, USA, 1964. [Google Scholar]
- McMurry, J.E. Organic Chemistry, 6th ed.; Brooks Cole: Monterey, CA, USA, 2003. [Google Scholar]
- Al-Busaidi, I.K.; Al-Maamari, R.S.; Karimi, M.; Naser, J. Effect of different polar organic compounds on wettability of calcite surfaces. J. Petrol. Sci. Eng. 2019, 180, 569–583. [Google Scholar] [CrossRef]
- Xie, Z.; Jiang, H.; Sun, Z.; Yang, Q. Direct AFM measurements of morphology and interaction force at solid-liquid interfaces between DTAC/CTAC and mica. J. Cent. South Univ. 2016, 23, 2182–2190. [Google Scholar] [CrossRef]
- Chennakesavulu, K.; Raju, G.B.; Prabhakar, S.; Nair, C.M.; Murthy, K.V.G.K. Adsorption of oleate on fluorite surface as revealed by atomic force microscopy. Int. J. Miner. Process. 2009, 90, 101–104. [Google Scholar] [CrossRef]














| C18 Fatty Acid | Structure and Degree of Saturation | Theoretically Calculated Length/nm | Limiting Area of Monolayer (Å2) | Intermolecular Distance in Monolayer (Å) | Melting Temperature (℃) a |
|---|---|---|---|---|---|
| SA | 18:0 | 2.62–2.66 [36] | 20 [36,37] | 4.47 [38] | 69–71 |
| OA | 18:1; (cis)9 | 2.48–2.52 [36] | 41 [36,39] | 6.40 [38] | 13–14 |
| LA | 18:2; (cis)9,12 | 2.40–2.44 [36] | 48 [36,40] | 6.93 [38] | −5~−1 |
| ALA | 18:3; (cis)9,12,15 | -b | -b | -b | −11~−10 |
| Fatty Acid | Premicellar Concentration (mol/L) | CMC (mol/L) | γCMC (mN/m) |
|---|---|---|---|
| SA | 1 × 10−6 | 1 × 10−4 | 33.915 |
| OA | 1 × 10−5 | 1 × 10−3 | 24.298 |
| LA | 1 × 10−5 | 1 × 10−3 | 29.298 |
| ALA | 1 × 10−5 | 1 × 10−3 | 30.631 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Z.; Zhang, Q.; Li, X.; Chen, Q. Adsorption Behavior and Wettability of Rhodochrosite Surface: Effect of C18 Fatty Acid Unsaturation. Minerals 2020, 10, 905. https://doi.org/10.3390/min10100905
Shen Z, Zhang Q, Li X, Chen Q. Adsorption Behavior and Wettability of Rhodochrosite Surface: Effect of C18 Fatty Acid Unsaturation. Minerals. 2020; 10(10):905. https://doi.org/10.3390/min10100905
Chicago/Turabian StyleShen, Zhihui, Qin Zhang, Xianbo Li, and Qianlin Chen. 2020. "Adsorption Behavior and Wettability of Rhodochrosite Surface: Effect of C18 Fatty Acid Unsaturation" Minerals 10, no. 10: 905. https://doi.org/10.3390/min10100905