Smectitization as a Trigger of Bacterially Mediated Mn-Fe Micronodule Generation in Felsic Glass (Livno-Tomislavgrad Paleolake, Bosnia and Herzegovina)
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Description and Eogenetic Evolution of Volcanic Tuff
4.2. SEM-EDS Mineralogy and Morphology of Mn-Fe Micronodules
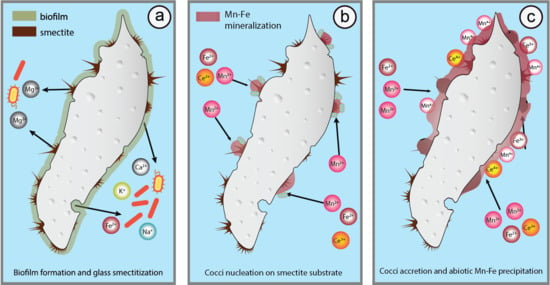
5. Discussion
5.1. Microbially Mediated Glass Transformations
5.2. Bacterial Textures in Mn-Fe Miconodules
5.3. Glass Smectitization as a Trigger of Mn-Fe Precipitation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, D.G.; Fisher, T.G. Glacial Lake Agassiz: The northwestern outlet and paleoflood. Geology 1993, 21, 9–12. [Google Scholar] [CrossRef]
- Sohn, R.A.; Sims, K.W.W. Bending as a mechanism for triggering off-axis volcanism on the East Pacific Rise. Geology 2005, 33, 93–96. [Google Scholar] [CrossRef]
- Marsaglia, K.M.; Tazaki, K. Diagenetic trends in Leg 126 sandstones. Proc. Ocean Drill. Prog. Sci. Results 1992, 126, 125–138. [Google Scholar]
- Bohor, B.F.; Triplehorn, D.M. Tonsteins: Altered Volcanic Ash Layers in Coal-Bearing Sequences; Geological Society of America: Boulder, CO, USA, 1993; Volume 285, ISBN 0813722853. [Google Scholar]
- Gifkins, C.C.; Herrmann, W.; Large, R.R. Altered Volcanic Rocks: A Guide to Description and Interpretation; Centre for Ore Deposit Research, University of Tasmania: Hobart, Australia, 2005. [Google Scholar]
- Lipman, P.W. Chemical comparison of glassy and crystalline volcanic rocks. Bull. U.S. Geol. Soc. 1965, 1201, 24. [Google Scholar]
- Fisher, R.V.; Schmincke, H.U. Pyroclastic Rocks; Springer: Berlin/Heidelberg, Germany, 1984; ISBN 3540127569. [Google Scholar]
- Casey, W.H.; Bunker, B. Leaching of mineral and glass surfaces during dissolution. Rev. Mineral. Geochem. 1990, 23, 397–426. [Google Scholar]
- Reich, V.; Von Rad, U. Eocene porcellanites and early Cretaceous cherts from the western North Atlantic basin. Initial Rep. Deep Sea Drill. Proj. 1979, 43, 437–448. [Google Scholar]
- Aoyagi, K.; Kazama, T. Transformational changes of clay minerals, zeolites and silica minerals during diagenesis. Sedimentology 1980, 27, 179–188. [Google Scholar] [CrossRef]
- Broxton, D.E.; Bish, D.L.; Warren, R.G. Distribution and chemistry of diagenetic minerals at Yucca Mountain, Nye County, Nevada. Clays Clay Miner. 1987, 35, 89–110. [Google Scholar] [CrossRef]
- Cuadros, J.; Afsin, B.; Jadubansa, P.; Ardakani, M.; Ascaso, C.; Wierzchos, J.; Adams, J. Pathways of volcanic glass alteration in laboratory experiments through inorganic and microbially-mediated processes. Clay Miner. 2013, 48, 423–445. [Google Scholar] [CrossRef]
- Cuadros, J. Clay minerals interaction with microorganisms: A review. Clay Miner. 2017, 52, 235–262. [Google Scholar] [CrossRef] [Green Version]
- Staudigel, H.; Furnes, H.; McLoughlin, N.; Banerjee, N.R.; Connell, L.B.; Templeton, A. 3.5 billion years of glass bioalteration: Volcanic rocks as a basis for microbial life? Earth Sci. Rev. 2008, 89, 156–176. [Google Scholar] [CrossRef]
- Cockell, C.S.; Herrera, A. Why are some microorganisms boring? Trends Microbiol. 2008, 16, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Cockell, C.S.; Olsson-Francis, K.; Herrera, A.; Meunier, A. Alteration textures in terrestrial volcanic glass and the associated bacterial community. Geobiology 2009, 7, 50–65. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.; Cockell, C.S.; Self, S.; Blaxter, M.; Reitner, J.; Arp, G.; Dröse, W.; Thorsteinsson, T.; Tindle, A. Bacterial colonization and weathering of terrestrial obsidian in Iceland. Geomicrobiol. J. 2008, 25, 25–37. [Google Scholar] [CrossRef]
- McLoughlin, N.; Furnes, H.; Banerjee, N.R.; Staudigel, H.; Muehlenbachs, K.; De Wit, M.; Van Kranendonk, M.J. Micro-bioerosion in volcanic glass: Extending the ichnofossil record to Archaean basaltic crust. In Current Developments in Bioerosion; Springer: Berlin/Heidelberg, Germany, 2008; pp. 371–396. [Google Scholar]
- Staudigel, H.; Chastain, R.A.; Yayanos, A.; Bourcier, W. Biologically mediated dissolution of glass. Chem. Geol. 1995, 126, 147–154. [Google Scholar] [CrossRef]
- Thorseth, I.H.; Furnes, H.; Tumyr, O. Textural and chemical effects of bacterial activity on basaltic glass: An experimental approach. Chem. Geol. 1995, 119, 139–160. [Google Scholar] [CrossRef]
- Fisk, M.R.; Giovannoni, S.J.; Thorseth, I.H. Alteration of oceanic volcanic glass: Textural evidence of microbial activity. Science 1998, 281, 978–980. [Google Scholar] [CrossRef]
- Sánchez-Navas, A.; Martín Algarra, A.; Nieto, F. Bacterially-Mediated Authigenesis of clays in Phosphate Stromatolites. Sedimentology 1998, 45, 519–533. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Urrutia, M.M. Bacterial clay authigenesis: A common biogeochemical process. Chem. Geol. 1999, 161, 399–413. [Google Scholar] [CrossRef]
- De Leeuw, A.; Mandic, O.; Krijgsman, W.; Kuiper, K.; Hrvatović, H. A chronostratigraphy for the Dinaride Lake System deposits of the Livno-Tomislavgrad Basin: The rise and fall of a long-lived lacustrine environment. Stratigraphy 2011, 8, 29–43. [Google Scholar] [CrossRef]
- Mandic, O.; Sant, K.; Kallanxhi, M.-E.; Ćorić, S.; Theobalt, D.; Grunert, P.; de Leeuw, A.; Krijgsman, W. Integrated bio-magnetostratigraphy of the Badenian reference section Ugljevik in southern Pannonian Basin-implications for the Paratethys history (middle Miocene, Central Europe). Glob. Planet. Chang. 2019, 172, 374–395. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef] [Green Version]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L.; Newman, D.K.; Kappler, A. Ehrlich’s Geomicrobiology; CRC Press: Boca Raton, FL, USA, 2015; ISBN 1466592419. [Google Scholar]
- Stein, L.Y.; La Duc, M.T.; Grund, T.J.; Nealson, K.H. Bacterial and archaeal populations associated with freshwater ferromanganous micronodules and sediments. Environ. Microbiol. 2001, 3, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sommers, M.G.; Dollhopf, M.E.; Douglas, S. Freshwater ferromanganese stromatolites from lake vermilion, Minnesota: Microbial culturing and environmental scanning electron microscopy investigations. Geomicrobiol. J. 2002, 19, 407–427. [Google Scholar] [CrossRef]
- Owen, R.B.; Renaut, R.W.; Williams, T.M. Characteristics and origins of laminated ferromanganese nodules from Lake Malawi, Central Africa. In Limnology, Climatology and Paleoclimatology of the East African Lakes; Routledge: Abingdon, UK, 2019; pp. 461–474. [Google Scholar]
- Gillette, N.J. Oneida Lake pancakes. N. Y. State Conserv. 1961, 18, 41. [Google Scholar]
- Saratovsky, I.; Gurr, S.J.; Hayward, M.A. The structure of manganese oxide formed by the fungus Acremonium sp. strain KR21-2. Geochim. Cosmochim. Acta 2009, 73, 3291–3300. [Google Scholar] [CrossRef]
- Spiro, T.G.; Bargar, J.R.; Sposito, G.; Tebo, B.M. Bacteriogenic manganese oxides. Acc. Chem. Res. 2010, 43, 2–9. [Google Scholar] [CrossRef]
- Schweitzer-Chaput, B.; Horwitz, M.A.; de Pedro Beato, E.; Melchiorre, P. Photochemical generation of radicals from alkyl electrophiles using a nucleophilic organic catalyst. Nat. Chem. 2019, 11, 129–135. [Google Scholar] [CrossRef]
- Tari, V. Evolution of the northern and western Dinarides: A tectonostratigraphic approach. In Continental Collision and the Tectono-Sedimentary Evolution of Forelands; Copernicus: Göttingen, Germany, 2002; ISBN 3936586004. [Google Scholar]
- Schmid, S.M.; Bernoulli, D.; Fügenschuh, B.; Matenco, L.; Schefer, S.; Schuster, R.; Tischler, M.; Ustaszewski, K. The Alpine-Carpathian-Dinaridic orogenic system: Correlation and evolution of tectonic units. Swiss J. Geosci. 2008, 101, 139–183. [Google Scholar] [CrossRef] [Green Version]
- Tari, V.; Pamić, J. Geodynamic evolution of the northern Dinarides and the southern part of the Pannonian Basin. Tectonophysics 1998, 297, 269–281. [Google Scholar] [CrossRef]
- Korbar, T. Orogenic evolution of the External Dinarides in the NE Adriatic region: A model constrained by tectonostratigraphy of Upper Cretaceous to Paleogene carbonates. Earth Sci. Rev. 2009, 96, 296–312. [Google Scholar] [CrossRef]
- De Leeuw, A.; Mandic, O.; Krijgsman, W.; Kuiper, K.; Hrvatović, H. Paleomagnetic and geochronologic constraints on the geodynamic evolution of the Central Dinarides. Tectonophysics 2012, 530, 286–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Unen, M.; Matenco, L.; Nader, F.H.; Darnault, R.; Mandic, O.; Demir, V. Kinematics of foreland-vergent crustal accretion: Inferences from the Dinarides evolution. Tectonics 2019, 38, 49–76. [Google Scholar] [CrossRef]
- Van Unen, M.; Matenco, L.; Demir, V.; Nader, F.H.; Darnault, R.; Mandic, O. Transfer of deformation during indentation: Inferences from the post-middle Miocene evolution of the Dinarides. Glob. Planet. Change 2019, 182, 103027. [Google Scholar] [CrossRef]
- Andrić, N.; Sant, K.; Matenco, L.; Mandic, O.; Tomljenović, B.; Pavelić, D.; Hrvatović, H.; Demir, V.; Ooms, J. The link between tectonics and sedimentation in asymmetric extensional basins: Inferences from the study of the Sarajevo-Zenica Basin. Mar. Pet. Geol. 2017, 83, 305–332. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Moreno, G.; Mandic, O.; Harzhauser, M.; Pavelić, D.; Vranjković, A. Vegetation and climate dynamics during the early middle Miocene from Lake Sinj (Dinaride Lake system, SE Croatia). Rev. Palaeobot. Palynol. 2008, 152, 237–245. [Google Scholar]
- Krstić, N.; Dumurdzanov, N.; Jankonić-Golubović, J.; Vujnović, L.; Olujić, J. Interbedded tuff and bentonite in the Neogene lacustrine sediments of the Balkan Peninsula. A review. J. Natl. Volcan. Group Italy 2001, 13, 1000–1009. [Google Scholar]
- Hrvatović, H. Geological Guidebook Through Bosnia and Herzegovina; Geological Survey of Bosnia and Herzegovina: Sarajevo, Bosnia and Herzegovina, 2006. [Google Scholar]
- De Leeuw, J.H.W.M. Paleomagnetic and geochronologic constraints on the Miocene evolution of semi-isolated basins in southeastern Europe. Ph.D. Thesis, University of Utrecht, Utrecht, The Netherlands, 2011. [Google Scholar]
- Ritchie, N.W.M. Getting Started with NIST DTSA-II. Micros. Today 2011, 19, 26–31. [Google Scholar] [CrossRef]
- Thorseth, I.H.; Pedersen, R.B.; Christie, D.M. Microbial alteration of 0-30-Ma seafloor and sub-seafloor basaltic glasses from the Australian Antarctic Discordance. Earth Planet. Sci. Lett. 2003, 215, 237–247. [Google Scholar] [CrossRef]
- Wang, X.; Gan, L.; Wiens, M.; Schloßmacher, U.; Schröder, H.C.; Müller, W.E.G. Distribution of Microfossils Within Polymetallic Nodules: Biogenic Clusters Within Manganese Layers. Mar. Biotechnol. 2012, 14, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Brown, G. Crystal Structures of Clay Minerals and Their X-ray Identification; The Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 1982; Volume 5, ISBN 0903056089. [Google Scholar]
- Bardossy, G.; Brindley, G.W. Rancieite associated with a karstic bauxite deposit. Am. Mineral. 1978, 63, 762–767. [Google Scholar]
- Sileo, E.E.; Alvarez, M.; Rueda, E.H. Structural studies on the manganese for iron substitution in the synthetic goethite—Jacobsite system. Int. J. Inorg. Mater. 2001, 3, 271–279. [Google Scholar] [CrossRef]
- Heiken, G.; Wohletz, K. Volcanic Ash; University Presses of California, Chicago, Harvard & MIT: Oakland, CA, USA, 1985; ISBN 0520052412. [Google Scholar]
- Walker, G.P.L. Plinian eruptions and their products. Bull. Volcanol. 1981, 44, 223–240. [Google Scholar] [CrossRef]
- Guven, N. Smectites. Reviews in Mineralogy. In Hydrous Phyllosilicates; Bailey, S.W., Ed.; Mineralogical Society of America: Washington, DC, USA, 1988; Volume 19, pp. 497–559. [Google Scholar]
- Ddani, M.; Meunier, A.; Zahraoui, M.; Beaufort, D.; El Wartiti, M.; Fontaine, C.; Boukili, B.; El Mahi, B. Clay mineralogy and chemical composition of bentonites from the Gourougou volcanic massif (northeast Morocco). Clays Clay Miner. 2005, 53, 250–267. [Google Scholar] [CrossRef]
- Giovannoni, S.J.; Fisk, M.R.; Mullins, T.D.; Furnes, H. Genetic Evidence for Endolithic Microbial Life Colonizing Basaltic Glass/Seawater Interfaces. Proc. Ocean Drill. Prog. Sci. Results 1996, 148. [Google Scholar] [CrossRef]
- Furnes, H.; Staudigel, H.; Thorseth, I.H.; Torsvik, T.; Muehlenbachs, K.; Tumyr, O. Bioalteration of basaltic glass in the oceanic crust. Geochem. Geophys. Geosyst. 2001, 2, 2000GC000150. [Google Scholar] [CrossRef]
- Joy, D.C. Monte Carlo Modeling for Electron Microscopy and Microanalysis; Oxford University Press: Oxford, UK, 1995; ISBN 0195358465. [Google Scholar]
- Fesharaki, O.; García-Romero, E.; Cuevas-González, J.; López-Martínez, N. Clay mineral genesis and chemical evolution in the Miocene sediments of Somosaguas, Madrid Basin, Spain. Clay Miner. 2007, 42, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Kepkay, P.E. Kinetics of microbial manganese oxidation and trace metal binding in sediments: Results from an in situ dialysis technique. Limnol. Oceanogr. 1985, 30, 713–726. [Google Scholar] [CrossRef]
- Gasparatos, D.; Massas, I.; Godelitsas, A. Fe-Mn concretions and nodules formation in redoximorphic soils and their role on soil phosphorus dynamics: Current knowledge and gaps. Catena 2019, 182, 104106. [Google Scholar] [CrossRef]
- Ram, H.; Singh, R.P.; Prasad, J. Chemical and mineralogical composition of Fe-Mn concretions and calcretes occurring in sodic soils of Eastern Uttar Pradesh, India. Soil Res. 2001, 39, 641–648. [Google Scholar] [CrossRef]
- Šegvić, B.; Girardclos, S.; Zanoni, G.; Gonzalez, C.A.; Steimer-Herbet, T.; Besse, M. Origin and paleoenvironmental significance of Fe–Mn nodules in the Holocene perialpine sediments of Geneva Basin, western Switzerland. Appl. Clay Sci. 2018, 160, 22–39. [Google Scholar] [CrossRef]
- Dubinin, A.V.; Sval’nov, V.N.; Berezhnaya, E.D.; Rimskaya-Korsakova, M.N.; Demidova, T.P. Geochemistry of trace and minor elements in sediments and manganese micronodules from the Angola Basin. Lithol. Miner. Resour. 2013, 48, 175–197. [Google Scholar] [CrossRef]
- Wang, X.; Schloßmacher, U.; Wiens, M.; Schröder, H.C.; Müller, W.E.G. Biogenic origin of polymetallic nodules from the Clarion-Clipperton Zone in the Eastern Pacific Ocean: Electron microscopic and EDX evidence. Mar. Biotechnol. 2009, 11, 99–108. [Google Scholar] [CrossRef]
- Pattan, J.N. Manganese micronodules: A possible indicator of sedimentary environments. Mar. Geol. 1993, 113, 331–344. [Google Scholar] [CrossRef]
- Rothe, J.; Kneedler, E.M.; Pecher, K.; Tonner, B.P.; Nealson, K.H.; Grundl, T.; Meyer-Ilse, W.; Warwick, T. Spectromicroscopy of Mn distributions in micronodules produced by biomineralization. J. Synchrotron Radiat. 1999, 6, 359–361. [Google Scholar] [CrossRef] [Green Version]
- Mandernack, K.W.; Post, J.; Tebo, B.M. Manganese mineral formation by bacterial spores of the marine Bacillus, strain SG-1: Evidence for the direct oxidation of Mn(II) to Mn(IV). Geochim. Cosmochim. Acta 1995, 59, 4393–4408. [Google Scholar] [CrossRef]
- Potter, R.M.; Rossman, G.R. Mineralogy of manganese dendrites and coatings. Am. Miner. 1979, 64, 1219–1226. [Google Scholar]
- McKeown, D.A.; Post, J.E. Characterization of manganese oxide mineralogy in rock varnish and dendrites using X-ray absorption spectroscopy. Am. Mineral. 2001, 86, 701–713. [Google Scholar] [CrossRef]
- Lugović, B.; Šegvić, B.; Šegvić, T. Mn-crust todorokite mineralization on SW backshore Cretaceous limestones from the island of Dugi Otok (Central Adriatic, Croatia). Acta Adriat. 2008, 49, 53–63. [Google Scholar]
- von Stackelberg, U. Manganese nodules of the Peru Basin. Handb. Mar. Miner. Depos. 2000, 197–238. [Google Scholar]
- Marchig, V.; Von Stackelberg, U.; Hufnagel, H.; Durn, G. Compositional changes of surface sediments and variability of manganese nodules in the Peru Basin. Deep. Res. Part II Top. Stud. Oceanogr. 2001, 48, 3523–3547. [Google Scholar] [CrossRef]
- Cronan, D.S. Manganese Nodules, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128130810. [Google Scholar]
- Feng, X.H.; Zhu, M.; Ginder-Vogel, M.; Ni, C.; Parikh, S.J.; Sparks, D.L. Formation of nano-crystalline todorokite from biogenic Mn oxides. Geochim. Cosmochim. Acta 2010, 74, 3232–3245. [Google Scholar] [CrossRef]
- MacRae, N.D.; Nesbitt, H.W.; Kronberg, B.I. Development of a positive Eu anomaly during diagenesis. Earth Planet. Sci. Lett. 1992, 109, 585–591. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Johannesson, K.H. Rare earth elements (REE) and yttrium in stream waters, stream sediments, and Fe-Mn oxyhydroxides: Fractionation, speciation, and controls over REE + Y patterns in the surface environment. Geochim. Cosmochim. Acta 2008, 72, 5962–5983. [Google Scholar] [CrossRef]
- German, C.R.; Elderfield, H. Rare earth elements in Saanich Inlet, British Columbia, a seasonally anoxic basin. Geochim. Cosmochim. Acta 1989, 53, 2561–2571. [Google Scholar] [CrossRef]
- Hawkins, D.B.; Roy, R. Experimental hydrothermal studies on rock alteration and clay mineral formation. Geochim. Cosmochim. Acta 1963, 27, 1047–1054. [Google Scholar] [CrossRef]
- Wolff-Boenisch, D.; Gislason, S.R.; Oelkers, E.H.; Putnis, C. V The dissolution rates of natural glasses as a function of their composition at pH 4 and 10.6, and temperatures from 25 to 74 C. Geochim. Cosmochim. Acta 2004, 68, 4843–4858. [Google Scholar] [CrossRef]
- Walton, A.W. Microtubules in basalt glass from Hawaii Scientific Driling Project #2 phase 1 core and Hilina slope, Hawaii: Evidence of the occurrence and behavior of endolithic microorganisms. Geobiology 2008, 6, 351–364. [Google Scholar] [CrossRef]
- Ménez, B.; Pasini, V.; Brunelli, D. Life in the hydrated suboceanic mantle. Nat. Geosci. 2012, 5, 133–137. [Google Scholar] [CrossRef]
- Furnes, H.; McLoughlin, N.; Muehlenbachs, K.; Banerjee, N.; Staudigel, H.; Dilek, Y.; De Wit, M.; Van Kranendonk, M.; Schiffman, P. Oceanic Pillow Lavas and Hyaloclastites as Habitats for Microbial Life Through Time—A Review. In Links Between Geological Processes, Microbial Activities & Evolution of Life: Microbes and Geology; Dilek, Y., Furnes, H., Muehlenbachs, K., Eds.; Modern Approaches in Solid Earth Sciences; Springer: Dordrecht, The Netherlands, 2008; pp. 1–68. ISBN 978-1-4020-8306-8. [Google Scholar]
- Kelly, L.C.; Cockell, C.S.; Piceno, Y.M.; Andersen, G.L.; Thorsteinsson, T.; Marteinsson, V. Bacterial diversity of weathered terrestrial Icelandic volcanic glasses. Microb. Ecol. 2010, 60, 740–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawano, M.; Tomita, K. Microbial biomineralization in weathered volcanic ash deposit and formation of biogenic minerals by experimental incubation. Am. Mineral. 2001, 86, 400–410. [Google Scholar] [CrossRef]
- Alimova, A.; Katz, A.; Steiner, N.; Rudolph, E.; Wei, H.; Steiner, J.C.; Gottlieb, P. Bacteria-clay interaction: Structural changes in smectite induced during biofilm formation. Clays Clay Miner. 2009, 57, 205–212. [Google Scholar] [CrossRef]
- Alimova, A.; Roberts, M.; Katz, A.; Rudolph, E.; Steiner, J.C.; Alfano, R.R.; Gottlieb, P. Effects of smectite clay on biofilm formation by microorganisms. Biofilms 2006, 3, 47–54. [Google Scholar] [CrossRef]
- Vieira, M.J.; Pacheco, A.P.; Pinho, I.A.; Melo, L.F. The effect of clay particles on the activity of suspended autotrophic nitrifying bacteria and on the performance of an air-lift reactor. Environ. Technol. 2001, 22, 123–135. [Google Scholar] [CrossRef]
- Krauskopf, K.B. Separation of manganese from iron in sedimentary processes. Geochim. Cosmochim. Acta 1957, 12, 61–84. [Google Scholar] [CrossRef]
- Zapffe, C. Deposition of manganese. Econ. Geol. 1931, 26, 799–832. [Google Scholar] [CrossRef]
- Biondi, J.C.; Polgári, M.; Gyollai, I.; Fintor, K.; Kovács, I.; Fekete, J.; Mojzsis, S.J. Biogenesis of the Neoproterozoic kremydilite manganese ores from Urucum (Brazil)—A new manganese ore type. Precambrian Res. 2020, 340. [Google Scholar] [CrossRef] [Green Version]
- Bargar, J.R.; Tebo, B.M.; Bergmann, U.; Webb, S.M.; Glatzel, P.; Chiu, V.Q.; Villalobos, M. Biotic and abiotic products of Mn (II) oxidation by spores of the marine Bacillus sp. strain SG-1. Am. Mineral. 2005, 90, 143–154. [Google Scholar] [CrossRef]
- Nayak, B.; Das, S.K.; Munda, P. Biogenic signature and ultra microfossils in ferromanganese nodules of the Central Indian Ocean Basin. J. Asian Earth Sci. 2013, 73, 296–305. [Google Scholar] [CrossRef]
- Hasle, G.R.; Syvertsen, E.E.; Steidinger, K.A.; Tangen, K.; Tomas, C.R. Identifying Marine Diatoms and Dinoflagellates; Elsevier: Amsterdam, The Netherlands, 1996; ISBN 0080534414. [Google Scholar]
- Pisera, A.; Siver, P.A.; Mandic, O. Miocene siliceous microfossils from the open cast coal mine Gračanica (Bugojno paleolake, Bosnia and Herzegovina) and their significance: A preliminary report. Paleobiodivers. Paleoenviron. 2020, 100, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Templeton, A.; Knowles, E. Microbial transformations of minerals and metals: Recent advances in geomicrobiology derived from synchrotron-based X-ray spectroscopy and X-ray microscopy. Annu. Rev. Earth Planet. Sci. 2009, 37, 367–391. [Google Scholar] [CrossRef]
- Nealson, K.H.; Tebo, B. Structural features of manganese precipitating bacteria. Orig. Life 1980, 10, 117–126. [Google Scholar] [CrossRef]
- Van Waasbergen, L.G.; Hildebrand, M.; Tebo, B.M. Identification and characterization of a gene cluster involved in manganese oxidation by spores of the marine Bacillus sp. strain SG-1. J. Bacteriol. 1996, 178, 3517–3530. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zeng, L.; Wiens, M.; Schloßmacher, U.; Jochum, K.P.; Schröder, H.C.; Müller, W.E.G. Evidence for a biogenic, microorganismal origin of rock varnish from the Gangdese Belt of Tibet. Micron 2011, 42, 401–411. [Google Scholar] [CrossRef]
- Ryan, K.J.; Ray, C.G. Medical Microbiology; McGraw Hill: New York, NY, USA, 2004; p. 370. [Google Scholar]
- Perdrial, J.N.; Warr, L.N.; Perdrial, N.; Lett, M.-C.; Elsass, F. Interaction between smectite and bacteria: Implications for bentonite as backfill material in the disposal of nuclear waste. Chem. Geol. 2009, 264, 281–294. [Google Scholar] [CrossRef]
- Iyer, S.D.; Sudhakar, M. Coexistence of pumice and manganese nodule fields—Evidence for submarine silicic volcanism in the Central Indian Basin. Deep Sea Res. Part I Oceanogr. Res. Pap. 1993, 40, 1123–1129. [Google Scholar] [CrossRef]






| An. | Type | SiO2 | Al2O3 | FeO | MgO | K2O | NaO | MnO | CaO | TiO2 | NiO | CeO2 | BaO | Sum | M/F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 92 | B-l | 41.1 | 11.6 | 14.6 | 5.7 | 5.1 | 16.1 | 3.1 | 2.5 | 99.8 | 1.10 | ||||
| 93 | B-l | 37.9 | 12.4 | 20.2 | 6.9 | 6.3 | 10.2 | 2.2 | 3.2 | 0.3 | 99.6 | 0.50 | |||
| 94 | B-l | 37.6 | 12.6 | 20.1 | 7.2 | 5.8 | 10.8 | 2.5 | 3.4 | 100 | 0.54 | ||||
| 96 | Bact. | 47.1 | 9.7 | 5.2 | 0.7 | 1.7 | 0.7 | 25.7 | 5.7 | 1.7 | 0.9 | 1.1 | 100.2 | 4.94 | |
| 97 | Bact. | 42.9 | 9.4 | 5.3 | 0.8 | 1.6 | 0.6 | 29.5 | 6.2 | 1.7 | 0.8 | 1.1 | 99.9 | 5.57 | |
| 98 | Band | 30.5 | 8.8 | 15.5 | 1.8 | 3.3 | 27.4 | 6.7 | 5 | 3.3 | 102.3 | 1.77 | |||
| 99 | Band | 29.5 | 7.9 | 13.9 | 0.8 | 2.5 | 30.6 | 6.7 | 5 | 3.1 | 100 | 2.20 | |||
| 100 | B-li | 32.7 | 10.9 | 17.9 | 5.3 | 4.9 | 19.8 | 4.3 | 3 | 0.6 | 99.4 | 1.11 | |||
| 101 | B-li | 29.4 | 10 | 16.3 | 5.3 | 4.4 | 25.7 | 4.4 | 2.6 | 0.5 | 1.5 | 100.1 | 1.58 | ||
| 102 | Bact. | 31.9 | 9.3 | 11.5 | 0.6 | 33 | 7.6 | 5.1 | 99 | 2.87 | |||||
| 75 | Altg | 58.1 | 11.9 | 4.8 | 0.7 | 2.3 | 0.7 | 15.1 | 3.4 | 0.9 | 0.8 | 99.7 | 3.15 | ||
| 76 | Altg | 58.9 | 11.8 | 4.5 | 0.7 | 1.9 | 0.8 | 15.3 | 3.2 | 0.7 | 0.8 | 99.4 | 3.40 | ||
| 77 | Altg | 56.3 | 11.1 | 4.6 | 0.8 | 2 | 0.6 | 17.9 | 3.3 | 0.6 | 0.6 | 0.9 | 99.8 | 3.89 | |
| 78 | Fgl | 76.3 | 12.5 | 1.8 | 3.5 | 1.2 | 3.4 | 1.3 | 100 | 1.89 | |||||
| 79 | Fgl | 72.7 | 12.7 | 2.3 | 0.3 | 3.1 | 1.2 | 5.2 | 1.9 | 0.4 | 99.8 | 2.26 | |||
| 80 | Tod | 19.6 | 10.3 | 2.2 | 2.6 | 0.7 | 0.6 | 51.3 | 6.3 | 5.5 | 1 | 100.1 | 23.32 | ||
| 81 | Tod | 18.6 | 12 | 3.6 | 1.4 | 0.5 | 0.6 | 50.6 | 6.5 | 0.9 | 5 | 99.7 | 14.06 | ||
| 83 | Fgl | 77.2 | 12.3 | 1.3 | 3.6 | 1.1 | 3.1 | 1.4 | 100 | 2.38 | |||||
| 86 | Tod | 25.4 | 8.6 | 3 | 1.3 | 0.9 | 0.6 | 47.8 | 6.6 | 0.4 | 4.5 | 0.9 | 100 | 15.93 | |
| 59 | Fgl | 71 | 12.4 | 2.5 | 2.9 | 1 | 6.4 | 2.8 | 0.9 | 99.9 | 2.56 | ||||
| 60 | Fgl | 67.3 | 13.3 | 2.9 | 2.6 | 0.6 | 8.3 | 3.1 | 1.1 | 99.2 | 2.86 | ||||
| 56 | Sme | 31.4 | 8.4 | 8.5 | 0.6 | 0.5 | 0.3 | 19.6 | 9.8 | 3.8 | 3 | 100.1 | 2.31 | ||
| 70 | Band | 36.3 | 8.4 | 8.9 | 1 | 28.6 | 8.9 | 4.7 | 3.1 | 99.9 | 3.21 | ||||
| 72 | Band | 36.2 | 9 | 8.1 | 0.7 | 27.6 | 9 | 5.2 | 3.9 | 99.7 | 3.41 | ||||
| 33p | Fgl | 79.7 | 13.4 | 1.6 | 4.1 | 0.2 | 0.9 | 99.9 | 0 | ||||||
| 34p | Sme | 74.2 | 17.6 | 2 | 1.5 | 3.1 | 1.3 | 0.3 | 100 | 0 | |||||
| 38p | MnC | 54.9 | 10.6 | 6.9 | 3.1 | 17 | 4.4 | 2.2 | 99.1 | 2.46 | |||||
| 40p | Sme | 71.1 | 18.5 | 1.7 | 1.8 | 2.3 | 0.7 | 0.3 | 1.4 | 0.3 | 100.1 | 0.18 | |||
| 44p | Sme | 73.7 | 17.6 | 2 | 1.7 | 2.6 | 0.4 | 0.5 | 1.3 | 0.3 | 100.1 | 0.25 | |||
| 50p | MnC | 58.1 | 12.2 | 5.6 | 0.4 | 2.3 | 0.3 | 15.1 | 3.9 | 1.9 | 99.8 | 2.70 | |||
| 51p | MnC | 40.7 | 10.1 | 3.8 | 0.8 | 1.5 | 0.3 | 33.8 | 5.2 | 1.8 | 0.7 | 1.2 | 99.9 | 8.89 | |
| 52p | MnC | 50 | 10.4 | 5.6 | 0.4 | 2.4 | 0.2 | 23.2 | 4.4 | 2 | 1.3 | 99.9 | 4.14 |
| Interpreted Phase | °2θ | d [Å] | Interpreted Phase | °2θ | d [Å] |
|---|---|---|---|---|---|
| smectite (Sm) | 6.02 | 14.65 | quartz (Qtz) | 20.87 | 4.25 |
| 19.79 | 4.48 | 26.65 | 3.34 | ||
| 40.24 | 2.23 | ||||
| sanidine (Sa) | 13.65 | 6.48 | 42.46 | 2.12 | |
| 23.78 | 3.73 | 50.15 | 1.81 | ||
| 27.46 | 3.24 | ||||
| 27.76 | 3.21 | tridymite (Trd) | 20.60 | 4.30 | |
| 41.68 | 2.16 | 35.85 | 2.50 | ||
| 13.79 | 6.41 | ||||
| cristobalite (Crs) | 21.99 | 4.03 | |||
| labradorite (Lab) | 15.71 | 5.63 | 31.51 | 2.83 | |
| 22.00 | 4.03 | 48.65 | 1.87 | ||
| 23.74 | 3.74 | ||||
| 24.3 | 3.65 | jacobsite (Jac) | 29.54 | 3.02 | |
| 27.77 | 3.2 | 34.77 | 2.58 | ||
| 28.05 | 3.17 | 42.42 | 2.09 | ||
| 30.65 | 2.91 | ranciéite (Ran) | 23.74 | 3.74 | |
| 31.49 | 2.83 | 35.89 | 2.49 | ||
| 51.06 | 1.76 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badurina, L.; Šegvić, B.; Mandic, O.; Zanoni, G. Smectitization as a Trigger of Bacterially Mediated Mn-Fe Micronodule Generation in Felsic Glass (Livno-Tomislavgrad Paleolake, Bosnia and Herzegovina). Minerals 2020, 10, 899. https://doi.org/10.3390/min10100899
Badurina L, Šegvić B, Mandic O, Zanoni G. Smectitization as a Trigger of Bacterially Mediated Mn-Fe Micronodule Generation in Felsic Glass (Livno-Tomislavgrad Paleolake, Bosnia and Herzegovina). Minerals. 2020; 10(10):899. https://doi.org/10.3390/min10100899
Chicago/Turabian StyleBadurina, Luka, Branimir Šegvić, Oleg Mandic, and Giovanni Zanoni. 2020. "Smectitization as a Trigger of Bacterially Mediated Mn-Fe Micronodule Generation in Felsic Glass (Livno-Tomislavgrad Paleolake, Bosnia and Herzegovina)" Minerals 10, no. 10: 899. https://doi.org/10.3390/min10100899