Brain Asymmetry: Towards an Asymmetrical Neurovisceral Integration
Abstract
:1. The Early Development
“Le cœur et le cerveau se trouvent dès lors dans une solidarité d’actions réciproques des plus intimes, qui se multiplient et se resserrent d’autant plus que l’organisme devient plus développé et plus délicat. Ces rapports peuvent être constants ou passagers, varier avec le sexe et avec l’âge”
“The molecular aspects of life reflect a complex system laced with feedback loops and multiple interactions-nothing is linear and simple”
2. Neurochemical Substrate for Brain Asymmetry
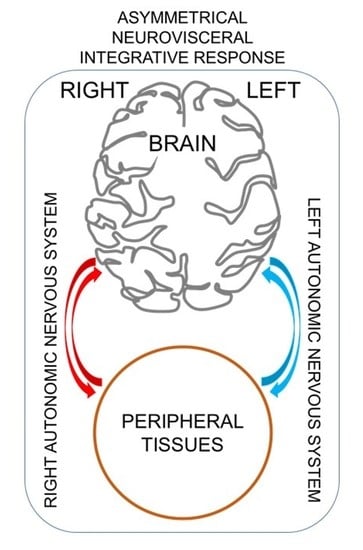
3. Asymmetric Neurovisceral Integration
4. Neuropathologies and Brain Asymmetry
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bernard, C. Étude sur la Physiologie du Cœur; Revue des Deux Mondes: Paris, France, 1878; pp. 316–366. [Google Scholar]
- Thayer, J.F.; Lane, R.D. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci. Biobehav. Rev. 2009, 33, 81–88. [Google Scholar] [CrossRef]
- Bernard, C. Étude sur la Physiologie du Cœur, 2nd ed.; Revue des Deux Mondes: Paris, France, 1865; Volume 56, pp. 236–252. [Google Scholar]
- Kucmierz, J.; Frak, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Molecular Interactions of Arterial Hypertension in Its Target Organs. Int. J. Mol. Sci. 2021, 22, 9669. [Google Scholar] [CrossRef]
- Segarra, A.B.; Prieto, I.; Banegas, I.; Martínez-Cañamero, M.; Villarejo, A.B.; Domínguez-Vías, G.; de Gasparo, M.; Ramírez-Sánchez, M. Interaction between Angiotensinase Activities in Pituitary and Adrenal Glands of Wistar-Kyoto and Spontaneously Hypertensive Rats under Hypotensive or Hypertensive Treatments. Int. J. Mol. Sci. 2021, 22, 7823. [Google Scholar] [CrossRef]
- Segarra, A.B.; Prieto, I.; Banegas, I.; Martinez-Cañamero, M.; de Gasparo, M.; Ramirez-Sanchez, M. Blood Pressure Correlates Asymmetrically with Neuropeptidase Activities of the Left and Right Frontal Cortices. Symmetry 2021, 13, 105. [Google Scholar] [CrossRef]
- Arias, J.A.; Williams, C.; Raghvani, R.; Aghajani, M.; Baez, S.; Belzung, C.; Booij, L.; Busatto, G.; Chiarella, J.; Fu, C.H.; et al. The neuroscience of sadness: A multidisciplinary synthesis and collaborative review. Neurosci. Biobehav. Rev. 2020, 111, 199–228. [Google Scholar] [CrossRef]
- Segarra, A.B.; Prieto-Gomez, I.; Banegas, I.; Martínez-Cañamero, M.; Luna, J.D.; de Gasparo, M.; Ramírez-Sánchez, M. Functional and neurometabolic asymmetry in SHR and WKY rats following vasoactive treatments. Sci. Rep. 2019, 9, 16098. [Google Scholar] [CrossRef] [PubMed]
- Balle, M.; Bornas, X.; Tortella-Feliu, M.; Llabrés, J.; Morillas-Romero, A.; Aguayo-Siquier, B.; Gelabert, J.M. Resting parietal EEG asymmetry and cardiac vagal tone predict attentional control. Biol. Psychol. 2013, 93, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M. The New Ambidextrous Universe; Dover Publications: New York, NY, USA, 2005; ISBN 0-486-44244-6. [Google Scholar]
- Corballis, M.C. Bilaterally Symmetrical: To Be or Not to Be? Symmetry 2020, 12, 326. [Google Scholar] [CrossRef] [Green Version]
- Corballis, M.C. How Asymmetries Evolved: Hearts, Brains, and Molecules. Symmetry 2021, 13, 914. [Google Scholar] [CrossRef]
- Hellige, J.B. Hemispheric Asymmetry. What’s Right and What’s Left; Harvard University Press: Cambridge, MA, USA, 1993; ISBN 0-674-38730-9. [Google Scholar]
- Rosen, J. Symmet.ry at the Foundation of Science and Nature. Symmetry 2009, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Rosen, J. Symmetry in Science. An introduction to the General Theory; Springer: New York, NY, USA, 1995; ISBN 0-387-94375-7. [Google Scholar]
- Close, F. Lucifer’s Legacy. The Meaning of Asymmetry; Oxford University Press: Oxford, UK, 2000; ISBN 0-19-850380-6. [Google Scholar]
- Gazzaniga, M.S. Tales from Both Sides of the Brain; HarperCollins Publishers: New York, NY, USA, 2015; ISBN 978-0-06-222880-2. [Google Scholar]
- Springer, S.P.; Deutchs, G. Left Brain, Right Brain; W.H. Freeman & Co Ltd.: New York, NY, USA, 1981; ISBN 9780716712695. [Google Scholar]
- McManus, C. Right Hand, Left Hand: The Origins of Asymmetry in Brains, Bodies, Atoms and Cultures; Harvard University Press: Cambridge, MA, USA, 2004; ISBN 978-0674016132. [Google Scholar]
- Nikolova, P.; Stoyanov, Z.; Negrev, N. Functional brain asymmetry, handedness and menarcheal age. Int. J. Psychophysiol. 1994, 18, 213–215. [Google Scholar] [CrossRef]
- Jones, R.E.; Desan, P.H.; Lopez, K.H.; Austin, H.B. Asymmetry in diencephalic monoamine metabolism is related to side of ovulation in a reptile. Brain Res. 1990, 506, 187–191. [Google Scholar] [CrossRef]
- Broca, P. Nouvelle observation d’aphémie par une lésion de la moitié postérieure des deuxième et troisième circonvolutions frontales. Bull. Soc. Anat. 1861, 6, 398–407. [Google Scholar]
- Wernicke, C. Der Aphasische Symptom Complex. Eine Psichologische Studie Ananatomischer Basis; Max Cohn & Wiegert: Breslau, Poland, 1874. [Google Scholar]
- Gazzaniga, M.S.; Bogen, J.E.; Sperry, R.W. Some functional effects of sectioning the cerebral commissures in man. Proc. Natl. Acad. Sci. USA 1962, 48, 1765–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Nobel Prize. Roger W. Sperry—Nobel Lecture. Available online: https://www.nobelprize.org/prizes/medicine/1981/sperry/25059-roger-w-sperry-nobel-lecture-1981 (accessed on 8 December 1981).
- Gazzaniga, M.S. One brain-two minds? Am. Sci. 1972, 60, 311–317. [Google Scholar]
- Corballis, M. Human Laterality; Academic Press: New York, NY, USA, 1983; ISBN 9780323158466. [Google Scholar]
- Oke, A.; Keller, R.; Mefford, I.; Adams, R.N. Lateralization of norepinephrine in human thalamus. Science 1978, 200, 1411–1413. [Google Scholar] [CrossRef]
- Oke, A.; Lewis, R.; Adams, R.N. Hemispheric asymmetry of norepinephrine distribution in rat thalamus. Brain Res. 1980, 188, 269–272. [Google Scholar] [CrossRef]
- Rosen, G.D.; Finklestein, S.; Stoll, A.L.; Yutzey, D.A.; Denenberg, V.H. Neurochemical asymmetries in the albino rat’s cortex, striatum, and nucleus accumbens. Life Sci. 1984, 34, 1143–1148. [Google Scholar] [CrossRef]
- Glick, S.D.; Ross, D.A.; Hough, L.B. Lateral asymmetry of neurotransmitters in human brain. Brain Res. 1982, 234, 53–63. [Google Scholar] [CrossRef]
- Shapiro, R.M.; Glick, S.D.; Hough, L.B. Striatal dopamine uptake asymmetries and rotational behavior in unlesioned rats: Revising the model? Psychopharmacology 1986, 89, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.M.; Crosley, K.J.; Keller, R.W.; Glick, S.D.; Carlson, J.N. Rotation, locomotor activity and individual differences in voluntary ethanol consumption. Brain Res. 1999, 823, 80–87. [Google Scholar] [CrossRef]
- Crapo, L.M. Hormones: The Messengers of Life; W H Freeman & Co.: New York, NY, USA, 1985; ISBN 0716717530, 9780716717539. [Google Scholar]
- Pert, C.B.; Snyder, S.H. Opiate receptor: Demonstration in nervous tissue. Science 1973, 179, 1011–1014. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Smith, T.W.; Kosterlitz, H.W.; Fothergill, L.A.; Morgan, B.A.; Morris, H.R. Identification of two related pentapeptides from the brain with potent opiate agonist activity. Nature 1975, 258, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Boler, J.; Enzmann, F.; Folkers, K.; Bowers, C.Y.; Schally, A.V. The identity of chemical and hormonal properties of the thyrotropin releasing hormone and pyroglutamyl-histidyl-proline amide. Biochem. Biophys. Res. Commun. 1969, 37, 705–710. [Google Scholar] [CrossRef]
- Burgus, R.; Dunn, T.F.; Desiderio, D.; Ward, D.N.; Vale, W.; Guillemin, R. Characterization of ovine hypothalamic hypophysiotropic TSH-releasing factor. Nature 1970, 226, 321–325, Erratum in Nature 1970, 226, 479. [Google Scholar] [CrossRef]
- Schwartz, J.C.; Roques, B.P. Opioid peptides as intercellular messengers. Biomedicine 1980, 32, 169–175. [Google Scholar] [PubMed]
- Grobecker, H. Transmitter-peptide coexistence in the central nervous system. Eur. Neurol. 1983, 22, 38–46. [Google Scholar] [CrossRef]
- Loh, Y.P.; Brownstein, M.J.; Gainer, H. Proteolysis in neuropeptide processing and other neural functions. Annu. Rev. Neurosci. 1984, 7, 189–222. [Google Scholar] [CrossRef] [PubMed]
- Merighi, A. Neuropeptides: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2011; ISSN 1064-3745. [Google Scholar]
- Vasilev, D.S.; Dubrovskaya, N.M.; Zhuravin, I.A.; Nalivaeva, N.N. Developmental Profile of Brain Neprilysin Expression Correlates with Olfactory Behaviour of Rats. J. Mol. Neurosci. 2021, 71, 1772–1785. [Google Scholar] [CrossRef]
- Ramírez, M.; Prieto, I.; Vives, F.; de Gasparo, M.; Alba, F. Neuropeptides, neuropeptidases and brain asymmetry. Curr. Protein Pept. Sci. 2004, 5, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Alba, F.; Ramirez, M.; Cantalejo, E.S.; Iribar, C. Aminopeptidase activity is asymmetrically distributed in selected zones of rat brain. Life Sci. 1988, 43, 935–939. [Google Scholar] [CrossRef]
- Banegas, I.; Prieto, I.; Alba, F.; Vives, F.; Araque, A.; Segarra, A.B.; Durán, R.; de Gasparo, M.; Ramírez, M. Angiotensinase activity is asymmetrically distributed in the amygdala, hippocampus and prefrontal cortex of the rat. Behav. Brain Res. 2005, 156, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Vías, G.; Aretxaga, G.; Prieto, I.; Segarra, A.B.; Luna, J.D.; Martínez-Cañamero, M.; Ramírez-Sánchez, M. Asymmetrical influence of a standard light/dark cycle and constant light conditions on the alanyl-aminopeptidase activity of the left and right retinas in adult male rats. Exp. Eye Res. 2020, 198, 108149. [Google Scholar] [CrossRef] [PubMed]
- Volchek, O.D. Vliianie tsiklichnosti sredy na proiavleniia funktsional’noĭ asimmetrii mozga u cheloveka [Effect of environmental cyclicity on appearance of human brain functional asymmetry]. Biofizika 1995, 40, 1013–1019. [Google Scholar]
- Agadzhanian, N.A.; Makarova, I.I.; Golovko, M.; D’iachkova, L.; Kanonidi, K. Elektrofiziologicheskiĭ i neĭrokhimicheskiĭ analiz biologicheskikh éffektov vozmushcheniĭ magnitnogo polia Zemli [Electrophysiological and neurochemical analysis of the biological effects of disturbances of Earth’s magnetic field]. Aviakosm. Ekolog. Med. 2002, 36, 26–32. [Google Scholar]
- Segarra, A.B.; Prieto, I.; Banegas, I.; Villarejo, A.B.; Wangensteen, R.; de Gasparo, M.; Vives, F.; Ramírez-Sánchez, M. The brain-heart connection: Frontal cortex and left ventricle angiotensinase activities in control and captopril-treated hypertensive rats-a bilateral study. Int. J. Hypertens. 2013, 2013, 156179. [Google Scholar] [CrossRef]
- Segarra, A.B.; Prieto, I.; Banegas, I.; Villarejo, A.B.; Wangensteen, R.; de Gasparo, M.; Vives, F.; Ramírez-Sánchez, M. Asymmetrical effect of captopril on the angiotensinase activity in frontal cortex and plasma of the spontaneously hypertensive rats: Expanding the model of neuroendocrine integration. Behav. Brain Res. 2012, 230, 423–427. [Google Scholar] [CrossRef]
- Banegas, I.; Prieto, I.; Segarra, A.B.; Durán, R.; Vives, F.; Alba, F.; Luna, J.D.; de Gasparo, M.; Wangesteen, R.; Ruiz-Bailén, M.; et al. Blood pressure increased dramatically in hypertensive rats after left hemisphere lesions with 6-hydroxydopamine. Neurosci. Lett. 2011, 500, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.F.; el-Salhy, M.; Danielsson, A.; Shalaby, A.; Axelsson, H. Changes in intestinal endocrine cells in the mouse after unilateral cervical vagotomy. Histol. Histopathol. 1999, 14, 453–460. [Google Scholar] [PubMed]
- Han, W.; Tellez, L.A.; Perkins, M.H.; Perez, I.O.; Qu, T.; Ferreira, J.; Ferreira, T.L.; Quinn, D.; Liu, Z.W.; Gao, X.B.; et al. A Neural Circuit for Gut-Induced Reward. Cell 2018, 175, 665–678. [Google Scholar] [CrossRef] [Green Version]
- Banegas, I.; Segarra, A.B.; Prieto, I.; Vives, F.; de Gasparo, M.; Duran, R.; de Dios Luna, J.; Ramírez-Sánchez, M. Asymmetrical response of aminopeptidase A in the medial prefrontal cortex and striatum of 6-OHDA-unilaterally-lesioned Wistar Kyoto and spontaneously hypertensive rats. Pharmacol. Biochem. Behav. 2019, 182, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Denenberg, V.H. Hemispheric laterality in animals and the effects of early experience. Behav. Brain Sci. 1981, 4, 1–49. [Google Scholar] [CrossRef] [Green Version]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains. The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: New York, NY, USA, 2013. [Google Scholar]
- Diz, D.I.; Ferrario, C.M. Bidirectional transport of angiotensin II binding sites in the vagus nerve. Hypertension 1988, 11, I139–I143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, C.O.; Coleman, M.P. KIF1A mediates axonal transport of BACE1 and identification of independently moving cargoes in living SCG neurons. Traffic 2016, 17, 1155–1167. [Google Scholar] [CrossRef]
- Hernández, J.; Prieto, I.; Segarra, A.B.; de Gasparo, M.; Wangensteen, R.; Villarejo, A.B.; Banegas, I.; Vives, F.; Cobo, J.; Ramírez-Sánchez, M. Interaction of neuropeptidase activities in cortico-limbic regions after acute restraint stress. Behav. Brain Res. 2015, 287, 42–48. [Google Scholar] [CrossRef]
- Segarra, A.B.; Hernández, J.; Prieto, I.; de Gasparo, M.; Ramírez-Sánchez, M. Neuropeptidase activities in plasma after acute restraint stress. Interaction with cortico-limbic areas. Acta Neuropsychiatr. 2016, 28, 239–243. [Google Scholar] [CrossRef]
- Knecht, S.; Dräger, B.; Deppe, M.; Bobe, L.; Lohmann, H.; Flöel, A.; Ringelstein, E.B.; Henningsen, H. Handedness and hemispheric language dominance in healthy humans. Brain 2000, 123, 2512–2518. [Google Scholar] [CrossRef] [Green Version]
- Lubben, N.; Ensink, E.; Coetzee, G.A.; Labrie, V. The enigma and implications of brain hemispheric asymmetry in neurodegenerative diseases. Brain Commun. 2021, 3, fcab211. [Google Scholar] [CrossRef]
- Ma, R.; Xie, Q.; Li, Y.; Chen, Z.; Ren, M.; Chen, H.; Li, H.; Li, J.; Wang, J. Animal models of cerebral ischemia: A review. Biomed. Pharmacother. 2020, 131, 110686. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, H.; Fang, Y.; Wang, S.; Zhou, H. The association between lesion location, sex and poststroke depression: Meta-analysis. Brain Behav. 2017, 7, e00788. [Google Scholar] [CrossRef]
- Hodgetts, S.; Hausmann, M. Antipsychotic effects of sex hormones and atypical hemispheric asymmetries. Cortex 2020, 127, 313–332. [Google Scholar] [CrossRef]
- Hausmann, M. Why sex hormones matter for neuroscience: A very short review on sex, sex hormones, and functional brain asymmetries. J. Neurosci. Res. 2017, 95, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Yarkoni, T.; Poldrack, R.A.; Nichols, T.E.; Van Essen, D.C.; Wager, T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods. 2011, 8, 665–670. [Google Scholar] [CrossRef] [Green Version]
- Bartolomeo, P.; Thiebaut de Schotten, M. Let thy left brain know what thy right brain doeth: Inter-hemispheric compensation of functional deficits after brain damage. Neuropsychologia 2016, 93, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toutain, T.G.L.O.; Alba, G.; Miranda, J.G.V.; do Rosário, R.S.; Munõz, M.; de Sena, E.P. Brain Asymmetry in Pain Affective Modulation. Pain Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Spernal, J.; Krieg, J.C.; Lautenbacher, S. Pain thresholds as a putative functional test for cerebral laterality in major depressive disorder and panic disorder. Neuropsychobiology 2003, 48, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Pauli, P.; Wiedemann, G.; Nickola, M. Pain sensitivity, cerebral laterality, and negative affect. Pain 1999, 80, 359–364. [Google Scholar] [CrossRef]
- Bolter, J.F.; Hannon, R. Lateralized cerebral dysfunction in early and late stage alcoholics. J. Stud. Alcohol 1986, 47, 213–218. [Google Scholar] [CrossRef]
- Nikolaeva, E.I.; Oteva, E.A.; Nikolaeva, A.A.; Shterental, I.S. Prognosis of myocardial infarction and brain functional asymmetry. Int. J. Cardiol. 1993, 42, 245–258. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Seidman, L.J.; O’Brien, L.M.; Horton, N.J.; Kennedy, D.N.; Makris, N.; Caviness, V.S.; Faraone, S.V.; Tsuang, M.T. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch. Gen. Psychiatry 2002, 59, 154–164. [Google Scholar] [CrossRef] [Green Version]
- Piras, F.; Cherubini, A.; Caltagirone, C.; Spalletta, G. Education mediates microstructural changes in bilateral hippocampus. Hum. Brain Mapp. 2011, 32, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Visser, T.A.; Ohan, J.L.; Whittle, S.; Yücel, M.; Simmons, J.G.; Allen, N.B. Sex differences in structural brain asymmetry predict overt aggression in early adolescents. Soc. Cogn. Affect. Neurosci. 2014, 9, 553–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutman, B.A.; van Erp, T.G.; Alpert, K.; Ching, C.R.; Isaev, D.; Ragothaman, A.; Jahanshad, N.; Saremi, A.; Zavaliangos-Petropulu, A.; Glahn, D.C.; et al. A meta-analysis of deep brain structural shape and asymmetry abnormalities in 2,833 individuals with schizophrenia compared with 3,929 healthy volunteers via the ENIGMA Consortium. Hum. Brain Mapp. 2021. [Google Scholar] [CrossRef]
- Marini, S.; Georgakis, M.K.; Anderson, C.D. Interactions Between Kidney Function and Cerebrovascular Disease: Vessel Pathology That Fires Together Wires Together. Front. Neurol. 2021, 12, 785273. [Google Scholar] [CrossRef]
- Viruega, H.; Gaviria, M. Functional Weight of Somatic and Cognitive Networks and Asymmetry of Compensatory Mechanisms: Collaboration or Divergency among Hemispheres after Cerebrovascular Accident? Life 2021, 11, 495. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Sánchez, M.; Prieto, I.; Segarra, A.B.; Banegas, I.; Martínez-Cañamero, M.; Domínguez-Vías, G.; de Gasparo, M. Brain Asymmetry: Towards an Asymmetrical Neurovisceral Integration. Symmetry 2021, 13, 2409. https://doi.org/10.3390/sym13122409
Ramírez-Sánchez M, Prieto I, Segarra AB, Banegas I, Martínez-Cañamero M, Domínguez-Vías G, de Gasparo M. Brain Asymmetry: Towards an Asymmetrical Neurovisceral Integration. Symmetry. 2021; 13(12):2409. https://doi.org/10.3390/sym13122409
Chicago/Turabian StyleRamírez-Sánchez, Manuel, Isabel Prieto, Ana Belén Segarra, Inmaculada Banegas, Magdalena Martínez-Cañamero, Germán Domínguez-Vías, and Marc de Gasparo. 2021. "Brain Asymmetry: Towards an Asymmetrical Neurovisceral Integration" Symmetry 13, no. 12: 2409. https://doi.org/10.3390/sym13122409