Pyrroloindole-Based Dynamic Combinatorial Chemistry
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Building Block Design and Properties
3.1.1. Synthesis
3.1.2. Optoelectronic and structural properties
3.2. Behaviour of l-PI in DCLs
3.2.1. DCLs of l-PI
3.2.2. Homochiral DCLs of l-PI and l-NDI
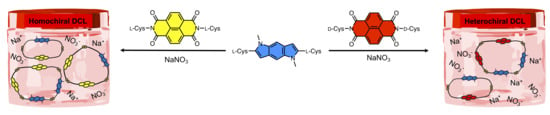
3.2.3. Heterochiral DCLs of l-PI and d-NDI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Corbett, P.T.; Leclaire, J.; Vial, L.; West, K.R.; Wietor, J.-L.; Sanders, J.K.; Otto, S. Dynamic combinatorial chemistry. Chem. Rev. 2006, 106, 3652–3711. [Google Scholar] [CrossRef]
- Moulin, E.; Cormos, G.; Giuseppone, N. Dynamic combinatorial chemistry as a tool for the design of functional materials and devices. Chem. Soc. Rev. 2012, 41, 1031–1049. [Google Scholar] [CrossRef] [PubMed]
- Buryak, A.; Severin, K. Dynamic combinatorial libraries of dye complexes as sensors. Angew. Chem. Int. Ed. 2005, 44, 7935–7938. [Google Scholar] [CrossRef] [PubMed]
- Carnall, J.M.A.; Waudby, C.A.; Belenguer, A.M.; Stuart, M.C.A.; Yang, X.; Peyralans, J.J.-P.; Otto, S. Mechanosensitive self-replication driven by self-organisation. Science 2010, 327, 1502–1506. [Google Scholar] [CrossRef] [PubMed]
- Malakoutikhah, M.; Peyralans, J.J.-P.; Colomb-Delsuc, M.; Fanlo-Virgós, H.; Stuart, M.C.A.; Otto, S. Uncovering the selection criteria for the emergence of multi-building-block replicators from dynamic combinatorial libraries. J. Am. Chem. Soc. 2013, 135, 18406–18417. [Google Scholar] [CrossRef]
- Li, J.; Nowak, P.; Otto, S. Dynamic combinatorial libraries: From exploring molecular recognition to systems chemistry. J. Am. Chem. Soc. 2013, 135, 9222–9239. [Google Scholar] [CrossRef]
- Sadownik, J.W.; Mattia, E.; Nowak, P.; Otto, S. Diversification of self-replicating molecules. Nat. Chem. 2016, 8, 264–269. [Google Scholar] [CrossRef]
- James, L.I.; Beaver, J.E.; Rice, N.W.; Waters, M.L. A synthetic receptor for asymmetric dimethyl arginine. J. Am. Chem. Soc. 2013, 135, 6450–6455. [Google Scholar] [CrossRef]
- Mullins, A.G.; Pinkin, N.K.; Hardin, J.A.; Waters, M.L. Achieving High affinity and selectivity for asymmetric Dimethylarginine by putting a lid on a box. Angew. Chem. Int. Ed. 2019, 58, 5282–5285. [Google Scholar] [CrossRef]
- Lam, R.T.S.; Belenguer, A.; Roberts, S.L.; Naumann, C.; Jarrosson, T.; Otto, S.; Sanders, J.K.M. Amplification of acetylcholine-binding catenanes from dynamic combinatorial libraries. Science 2005, 308, 667–669. [Google Scholar] [CrossRef]
- Bugaut, A.; Jantos, K.; Wietor, J.-L.; Rodriguez, R.; Sanders, J.K.M.; Balasubramanian, S. Exploring the differential recognition of DNA G-Quadruplex targets by small molecules using dynamic combinatorial chemistry. Angew. Chem. Int. Ed. 2008, 47, 2677–2680. [Google Scholar] [CrossRef] [PubMed]
- Cougnon, F.B.L.; Au-Yeung, H.Y.; Pantoş, G.D.; Sanders, J.K.M. Exploring the formation pathways of donor—Acceptor catenanes in aqueous dynamic combinatorial libraries. J. Am. Chem. Soc. 2011, 133, 3198–3207. [Google Scholar] [CrossRef] [PubMed]
- Dehkordi, M.E.; Luxami, V.; Pantoş, G.D. High-yielding synthesis of chiral donor—Acceptor catenanes. J. Org. Chem. 2018, 83, 11654–11660. [Google Scholar] [CrossRef] [PubMed]
- Ponnuswamy, N.; Cougnon, F.B.L.; Pantoş, G.D.; Sanders, J.K.M. Homochiral and meso figure eight knots and a Solomon link. J. Am. Chem. Soc. 2014, 136, 8243–8251. [Google Scholar] [CrossRef]
- Ponnuswamy, N.; Cougnon, F.B.L.; Clough, J.M.; Pantoş, G.D.; Sanders, J.K.M. Discovery of an organic trefoil knot. Science 2012, 338, 783–785. [Google Scholar] [CrossRef]
- Stefankiewicz, A.R.; Sambrook, M.R.; Sanders, J.K.M. Template-directed synthesis of multi-component organic cages in water. Chem. Sci. 2012, 3, 2326. [Google Scholar] [CrossRef]
- Stefankiewicz, A.R.; Sanders, J.K.M. Diverse topologies in dynamic combinatorial libraries from tri- and mono-thiols in water: Sensitivity to weak supramolecular interactions. Chem. Commun. 2013, 49, 5820. [Google Scholar] [CrossRef]
- Furusho, Y.; Oku, T.; Hasegawa, T.; Tsuboi, A.; Kihara, N.; Takata, T. Dynamic covalent approach to [2]- and [3]Rotaxanes by Utilizing a reversible Thiol–Disulfide interchange reaction. Chem.-Eur. J. 2003, 9, 2895–2903. [Google Scholar] [CrossRef]
- Kassem, S.; Lee, A.T.L.; Leigh, D.A.; Markevicius, A.; Solà, J. Pick-up, transport and release of a molecular cargo using a small-molecule robotic arm. Nat. Chem. 2015, 8, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Patrick, B.O.; Sherman, J.C. A new [4]carceplex, and a crystal structure and dynamic combinatorial chemistry of a [5]carceplex. Tetrahedron 2009, 65, 7296–7302. [Google Scholar] [CrossRef]
- Chichak, K.S.; Cantrill, S.J.; Pease, A.R.; Chiu, S.-H.; Cave, G.W.V.; Atwood, J.L.; Stoddart, J.F. Molecular borromean rings. Science 2004, 304, 1308–1312. [Google Scholar] [CrossRef]
- Meyer, C.D.; Forgan, R.S.; Chichak, K.S.; Peters, A.J.; Tangchaivang, N.; Cave, G.W.V.; Khan, S.I.; Cantrill, S.J.; Stoddart, J.F. The dynamic chemistry of molecular borromean rings and solomon knots. Chem.-Eur. J. 2010, 16, 12570–12581. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, C.D.; Chichak, K.S.; Peters, A.J.; Cave, G.W.V.; Cantrill, S.J.; Stoddart, J.F. A molecular Solomon link. Angew. Chem. Int. Ed. 2007, 46, 218–222. [Google Scholar] [CrossRef]
- Avestro, A.-J.; Gardner, D.M.; Vermeulen, N.A.; Wilson, E.A.; Schneebeli, S.T.; Whalley, A.C.; Belowich, M.E.; Carmieli, R.; Wasielewski, M.R.; Stoddart, J.F. Gated electron sharing within dynamic naphthalene Diimide-based Oligorotaxanes. Angew. Chem. Int. Ed. 2014, 53, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Belowich, M.E.; Valente, C.; Stoddart, J.F. Template-directed syntheses of rigid oligorotaxanes under thermodynamic control. Angew. Chem. Int. Ed. 2010, 49, 7208–7212. [Google Scholar] [CrossRef] [PubMed]
- Bilbeisi, R.A.; Ronson, T.K.; Nitschke, J.R. A self-assembled [Fe II 12 L 12] capsule with an icosahedral framework. Angew. Chem. Int. Ed. 2013, 52, 9027–9030. [Google Scholar] [CrossRef]
- Black, S.P.; Stefankiewicz, A.R.; Smulders, M.M.J.; Sattler, D.; Schalley, C.A.; Nitschke, J.R.; Sanders, J.K.M. Generation of a Dynamic system of three-dimensional tetrahedral Polycatenanes. Angew. Chem. Int. Ed. 2013, 52, 5749–5752. [Google Scholar] [CrossRef] [Green Version]
- Kieffer, M.; Pilgrim, B.S.; Ronson, T.K.; Roberts, D.A.; Aleksanyan, M.; Nitschke, J.R. Perfluorinated ligands induce Meridional metal stereochemistry to generate M8L12, M10L15, and M12L18 prisms. J. Am. Chem. Soc. 2016, 138, 6813–6821. [Google Scholar] [CrossRef] [Green Version]
- Jansze, S.M.; Cecot, G.; Wise, M.D.; Zhurov, K.O.; Ronson, T.K.; Castilla, A.M.; Finelli, A.; Pattison, P.; Solari, E.; Scopelliti, R.; et al. Ligand aspect ratio as a decisive factor for the self-assembly of coordination cages. J. Am. Chem. Soc. 2016, 138, 2046–2054. [Google Scholar] [CrossRef]
- Hasell, T.; Wu, X.; Jones, J.T.A.; Bacsa, J.; Steiner, A.; Mitra, T.; Trewin, A.; Adams, D.J.; Cooper, A.I. Triply interlocked covalent organic cages. Nat. Chem. 2010, 2, 750–755. [Google Scholar] [CrossRef]
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973–978. [Google Scholar] [CrossRef]
- Black, S.P.; Sanders, J.K.M.; Stefankiewicz, A.R. Disulfide exchange: Exposing supramolecular reactivity through dynamic covalent chemistry. Chem. Soc. Rev. 2014, 43, 1861–1872. [Google Scholar] [CrossRef] [PubMed]
- Cougnon, F.B.L.; Sanders, J.K.M. Evolution of dynamic combinatorial chemistry. Acc. Chem. Res. 2012, 45, 2211–2221. [Google Scholar] [CrossRef]
- Beeren, S.R.; Sanders, J.K.M. History and principles of dynamic combinatorial chemistry. In Dynamic Combinatorial Chemistry; Reek, J.N.H., Otto, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 1–22. [Google Scholar]
- Christinat, N.; Scopelliti, R.; Severin, K. Boron-based rotaxanes by multicomponent self-assembly. Chem. Commun. 2008, 31, 3660–3662. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; James, T.D.; Kubo, Y. Ion pair-driven Heterodimeric capsule based on Boronate esterification: Construction and the dynamic behavior. J. Am. Chem. Soc. 2007, 129, 15126–15127. [Google Scholar] [CrossRef]
- Nishimura, N.; Kobayashi, K. Self-assembly of a cavitand-based capsule by dynamic boronic ester formation. Angew. Chem. Int. Ed. 2008, 47, 6255–6258. [Google Scholar] [CrossRef] [PubMed]
- von Delius, M.; Geertsema, E.M.; Leigh, D.A. A synthetic small molecule that can walk down a track. Nat. Chem. 2010, 2, 96–101. [Google Scholar] [CrossRef] [PubMed]
- von Delius, M.; Geertsema, E.M.; Leigh, D.A.; Tang, D.-T.D. Design, synthesis, and operation of small molecules that walk along tracks. J. Am. Chem. Soc. 2010, 132, 16134–16145. [Google Scholar] [CrossRef] [PubMed]
- Pantoş, G.D.; Wietor, J.L.; Sanders, J.K.M. Filling helical nanotubes with C-60. Angew. Chem. Int. Ed. 2007, 46, 2238–2240. [Google Scholar] [CrossRef]
- Pantoş, G.D.; Pengo, P.; Sanders, J.K.M. Hydrogen-bonded helical organic nanotubes. Angew. Chem. Int. Ed. 2007, 46, 194–197. [Google Scholar] [CrossRef]
- Wietor, J.-L.; Pantoş, G.D.; Sanders, J.K.M. Templated amplification of an unexpected receptor for C70. Angew. Chem. Int. Ed. 2008, 47, 2689–2692. [Google Scholar] [CrossRef]
- Cacciapaglia, R.; Di Stefano, S.; Ercolani, G.; Mandolini, L. Combinatorial macrocyclizations under thermodynamic control: The two-monomer case. Macromolecules 2009, 42, 4077–4083. [Google Scholar] [CrossRef]
- Au-Yeung, H.Y.; Pantoş, G.D.; Sanders, J.K.M. Dynamic combinatorial donor−acceptor catenanes in water: Access to unconventional and unexpected structures. J. Org. Chem. 2011, 76, 1257–1268. [Google Scholar] [CrossRef]
- Au-Yeung, H.Y.; Pantoş, G.D.; Sanders, J.K.M. Dynamic combinatorial synthesis of a catenane based on donor-acceptor interactions in water. Proc. Natl. Acad. Sci. USA 2009, 106, 10466–10470. [Google Scholar] [CrossRef] [Green Version]
- Fass, D.; Thorpe, C. Chemistry and enzymology of disulfide cross-linking in proteins. Chem. Rev. 2018, 118, 1169–1198. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Luo, X.; Li, J.; Reed, S.A.; Xiao, H.; Young, T.S.; Schultz, P.G. Enhancing protein stability with extended disulfide bonds. Proc. Natl. Acad. Sci. USA 2016, 113, 5910–5915. [Google Scholar] [CrossRef] [Green Version]
- Bosnjak, I.; Bojovic, V.; Segvic-Bubic, T.; Bielen, A. Occurrence of protein disulfide bonds in different domains of life: A comparison of proteins from the Protein Data Bank. Protein Eng. Des. Sel. 2014, 27, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cougnon, F.B.L.; Ponnuswamy, N.; Jenkins, N.A.; Pantoş, G.D.; Sanders, J.K.M. Structural parameters Governing the dynamic combinatorial synthesis of catenanes in water. J. Am. Chem. Soc. 2012, 134, 19129–19135. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods VI: More modifications to the NDDO approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allouche, A.-R. Gabedit-A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Samsoniya, S.A.; Targamadze, N.L.; Suvorov, N.N. The chemistry of pyrroloindoles. Russ. Chem. Rev. 1994, 63, 815–832. [Google Scholar] [CrossRef]
- Manjal, S.K.; Pathania, S.; Bhatia, R.; Kaur, R.; Kumar, K.; Rawal, R.K. Diversified synthetic strategies for pyrroloindoles: An overview. J. Heterocycl. Chem. 2019, 56, 2318–2332. [Google Scholar] [CrossRef]
- Heaner, W.L., IV; Gelbaum, C.S.; Gelbaum, L.; Pollet, P.; Richman, K.W.; DuBay, W.; Butler, J.D.; Wells, G.; Liotta, C.L. Indoles via Knoevenagel–Hemetsberger reaction sequence. RSC Adv. 2013, 3, 13232–13242. [Google Scholar] [CrossRef]
- Tan, Y.; Ghandi, K. Kinetics and mechanism of pyrrole chemical polymerization. Synth. Met. 2013, 175, 183–191. [Google Scholar] [CrossRef]
- Donovalová, J.; Cigáň, M.; Stankovičová, H.; Gašpar, J.; Danko, M.; Gáplovský, A.; Hrdlovič, P. Spectral properties of substituted coumarins in solution and polymer matrices. Molecules 2012, 17, 3259–3276. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. The chaotropic effect as an assembly motif in chemistry. Angew. Chem. Int. Ed. 2018, 57, 13968–13981. [Google Scholar] [CrossRef] [Green Version]
- Gianga, T.-M. Topologically Complex Molecules: Synthesis and Properties. PhD Thesis, University of Bath, Bath, UK, 2020. [Google Scholar]
- Pengo, P.; Pantoş, G.D.; Otto, S.; Sanders, J.K.M. Efficient and mild microwave-assisted stepwise functionalization of Naphthalenediimide with α-amino acids. J. Org. Chem. 2006, 71, 7063–7066. [Google Scholar] [CrossRef] [Green Version]
- Au-Yeung, H.Y.; Pengo, P.; Pantoş, G.D.; Otto, S.; Sanders, J.K.M. Templated amplification of a naphthalenediimide-based receptor from a donor–acceptor dynamic combinatorial library in water. Chem. Commun. 2009, 4, 419–421. [Google Scholar] [CrossRef]








| Absorbance & CD | Emission | |||
|---|---|---|---|---|
| λmax (nm) | ε (L × mol−1 × cm−1) | Molar Ellipticity (deg × cm2 × dmol−1) | λmax (nm) | Φ (%) |
| 327 | 21,600 ± 1.8 | 2.11 × 104 | 394 | 48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gianga, T.-M.; Răsădean, D.-M.; Pantoș, G.D. Pyrroloindole-Based Dynamic Combinatorial Chemistry. Symmetry 2020, 12, 726. https://doi.org/10.3390/sym12050726
Gianga T-M, Răsădean D-M, Pantoș GD. Pyrroloindole-Based Dynamic Combinatorial Chemistry. Symmetry. 2020; 12(5):726. https://doi.org/10.3390/sym12050726
Chicago/Turabian StyleGianga, Tiberiu-Marius, Dora-Maria Răsădean, and G. Dan Pantoș. 2020. "Pyrroloindole-Based Dynamic Combinatorial Chemistry" Symmetry 12, no. 5: 726. https://doi.org/10.3390/sym12050726