Navigating Produced Water Sustainability in the Oil and Gas Sector: A Critical Review of Reuse Challenges, Treatment Technologies, and Prospects Ahead
Abstract
:1. Introduction
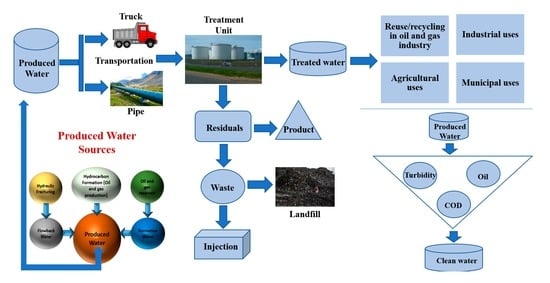
2. Overview of Produced Water in Oil and Gas Industry
3. Issues of Produced Water for the New Sustainability Challenge
4. Characteristics of Oilfield Produced Water
4.1. Physicochemical Characteristics
4.2. Inorganic Characteristics
4.3. Organic Characteristics
5. Produced Water Treatment Technologies
- (a)
- Elimination of fats and oils in free and dispersed states in PW;
- (b)
- Removal of organic matter dissolved in PW;
- (c)
- Elimination of different algae, bacteria, and microorganisms;
- (d)
- Haze separation by removing colloids and suspended matter;
- (e)
- Exclusion of gases dissolved in water;
- (f)
- Elimination of minerals and dissolved salts, leftover water hardness, and possible probable radioactive substances.
5.1. Physical Process
5.1.1. Hydrocyclone
5.1.2. Thermal Separation Process
5.1.3. Adsorption
5.1.4. Gravity Separation
5.1.5. Flotation
5.1.6. Coalescence Separation
5.1.7. Filtration Separation
5.2. Chemical Treatment
5.2.1. Chemical Precipitation
5.2.2. Chemical Oxidation
Electrochemical Oxidation Process
Electrodialysis (ED)
Photocatalytic Treatment
5.3. Thermal Treatment
| Methods | Target of Removal | Pros | Cons | Results | References |
|---|---|---|---|---|---|
| Chemical precipitation | Suspended and colloidal particles, hardness, heavy metals | (1) High recovery, simple operation, low cost, and energy-saving. (2) The pretreatment step is unnecessary | Secondary trash, sludge, chemical needs, metal-rich effluent | Excellent in cost saving | [8,189,195] |
| Chemical oxidation | Heavy metals, TDS, organics, BTEX, bacteria | Chemical-free, helpful secondary products, eco-friendly | (1) Scaling issues, expert labor needed (2) Process monitoring, optimization, pretreatment, and specialized labor are necessary for low-pollution wastewater | Environmentally friendly | [14,180,196] |
5.4. Biological Treatment
5.5. Membrane Treatment
5.6. Hybrid Technologies
6. Sustainable PW Management
6.1. Treatment Aspects
6.2. Regulatory Aspects
- Comply with the reporting obligations specified by the appropriate environmental agencies and obtain permits prior to discharging, injecting, or storing produced water. These include NPDES (National Pollutant Discharge Elimination System) and state-issued licenses, as well as UIC (Underground Injection Control) well permits;
- Conform with the criteria and standards for water quality that have been established by state and federal regulatory agencies. These standards establish the maximum allowable concentrations of various pollutants in the produced water and the bodies of water it may contaminate;
- Comply with standards governing the disposal and transportation of hazardous and non-hazardous waste generated during the treatment and handling of produced water. This may include following the Resource Conservation and Recovery Act (RCRA) regulations;
- Create and implement spill prevention and response plans to avoid inadvertent leaks of produced water or other contaminants. It is crucial to adhere to the regulations outlined in the Clean Water Act (CWA) and the Oil Pollution Act (OPA);
- Consistently monitor and provide regulatory authorities with reports on the quantity and quality of produced water, emissions, and discharges; frequently use electronic reporting systems. It is imperative to acquire the appropriate permits for UIC wells and adhere to the prescribed guidelines for injection wells, which may encompass pressure monitoring, mechanical integrity testing, and wellbore integrity assessments;
- In order to mitigate soil erosion and sediment discharge into water bodies, it is imperative to enforce erosion and sediment control measures mandated by regulatory agencies throughout the construction and operation phases. It is imperative to adhere to regulations pertaining to environmentally friendly completions and emission control, such as the Clean Air Act (CAA) and the implementation of best available control technology (BACT), to mitigate emissions;
- Ensure adherence to regulations pertaining to concentrated brine disposal and Zero Liquid Discharge (ZLD) systems, which may encompass standards for permits and disposal;
- Ensure that activities that have the potential to affect the environment or public health are duly communicated to the public and involve local stakeholders and communities in a manner consistent with regulatory requirements;
- Adhere to the stipulations placed forth by specific regulatory authorities with regard to the financing of research and development initiatives that seek to enhance technologies for water treatment and management;
- As required by federal and state agencies, conduct environmental impact assessments to determine the potential environmental effects of oilfield activities, such as produced water management;
- In order to verify compliance with relevant environmental regulations, regulatory authorities should conduct routine inspections and compliance assessments of the oilfield;
- Comply with standards governing the closure and remediation of oilfields, including produced water management facilities, to avoid long-term environmental damage.
7. Future Outlook of PW Treatment Technology
8. Conclusions
- The identification of constituents in PW makes it difficult to predict effective treatment methods. The scaling envelope and system performance for produced water are relatively unknown, making treatment technology selection difficult or ineffective. The market’s dynamic nature and new regulations increase the need for solutions to treat oilfield water, which has higher contaminants content. Off-the-shelf technologies can be developed for this purpose, driven by economics, flexibility, and real-time optimization. Analytical data are crucial for formulating treatment and optimization, but unreliable data are a concern as regulations tighten;
- Potentially sustainable PW is threatened by both nonconventional energy sources and an inadequate data repository. Contrary to common belief, shutting down oil and gas plants has resulted in a major reduction in water production. Based on WOR and limited test results, the actual volume of produced water is calculated. Oil and gas companies are hesitant to treat PW for beneficial reuse since meeting strict usage requirements is more expensive than simply disposing of it;
- PW’s complexity needs coordinated treatment to maximize water quality and save on costs. Thermal treatment cleans very polluted water, especially saline streams, with long life cycles. Membrane filtration technology has increased in popularity because it may solve conventional treatment methods’ high costs, harmful chemical use, requirement for specialized equipment and design, and undesired byproducts. Membrane filtering PW’s complex matrix requires advanced planning and more research on fouling solutions;
- Many physical, chemical, and biological approaches can comply with PW’s pollutants and reuse. However, PW’s complexity has prohibited separate technologies from converting it for reuse or disposal. More research is needed to determine the weighting factor of each component to the total risk, resulting in the best management plan;
- Chemical selection in oil and gas operations should consider non-organic carbon compounds, as removal is costly and difficult. Commercial treatment technologies are tailored to specific needs or compounds, making compact systems essential for sustainable treatment. These systems can address a wide range of pollutants while using minimal resources. No single technology can provide all desired effluent characteristics, so hybrid treatment systems may be used in series to meet regulatory limits. Environmental remediation purposes should be a key decision factor when choosing treatment technologies.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coday, B.D.; Xu, P.; Beaudry, E.G.; Herron, J.; Lampi, K.; Hancock, N.T.; Cath, T.Y. The sweet spot of forward osmosis: Treatment of produced water, drilling wastewater, and other complex and difficult liquid streams. Desalination 2014, 333, 23–35. [Google Scholar] [CrossRef]
- Coonrod, C.L.; Yin, Y.B.; Hanna, T.; Atkinson, A.J.; Alvarez, P.J.J.; Tekavec, T.N.; Reynolds, M.A.; Wong, M.S. Fit-for-purpose treatment goals for produced waters in shale oil and gas fields. Water Res. 2020, 173, 115467. [Google Scholar] [CrossRef] [PubMed]
- Jamaly, S.; Giwa, A.; Hasan, S.W. Recent improvements in oily wastewater treatment: Progress, challenges, and future opportunities. J. Environ. Sci. 2015, 37, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Souza, J.S.B.; Ferreira Júnior, J.M.; Simonelli, G.; Souza, J.R.; Góis, L.M.N.; Santos, L.C.L. Removal of oil contents and salinity from produced water using microemulsion. J. Water Process Eng. 2020, 38, 101548. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Wang, X.; Goual, L.; Colberg, P.J.S. Characterization and treatment of dissolved organic matter from oilfield produced waters. J. Hazard. Mater. 2012, 217–218, 164–170. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.; Biak, D.; Madaeni, S.; Abidin, Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, T.; Zhang, F. Evaluation of oilfield-produced water treated with a prepared magnetic inorganic polymer: Poly (silicate aluminum)/magnetite. J. Appl. Polym. Sci. 2018, 135, 45735. [Google Scholar] [CrossRef]
- Nasiri, M.; Jafari, I. Produced water from oil–gas plants: A short review on challenges and opportunities. Period. Polytech. Chem. Eng. 2017, 61, 73–81. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W. Challenges and trends in membrane technology implementation for produced water treatment: A review. J. Water Process Eng. 2014, 4, 107–133. [Google Scholar] [CrossRef]
- Alzahrani, S.; Mohammad, A.W.; Hilal, N.; Abdullah, P.; Jaafar, O. Comparative study of NF and RO membranes in the treatment of produced water II: Toxicity removal efficiency. Desalination 2013, 315, 27–32. [Google Scholar] [CrossRef]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, B.; Thanyamanta, W.; Hawboldt, K.; Zhang, B.; Liu, B. Offshore produced water management: A review of current practice and challenges in harsh/Arctic environments. Mar. Pollut. Bull. 2016, 104, 7–19. [Google Scholar] [CrossRef] [PubMed]
- da Silva, S.S.; Chiavone-Filho, O.; de Barros Neto, E.L.; Nascimento, C.A.O. Integration of processes induced air flotation and photo-Fenton for treatment of residual waters contaminated with xylene. J. Hazard. Mater. 2012, 199–200, 151–157. [Google Scholar] [CrossRef]
- Drewes, J.E.; Cath, T.; Xu, P.; Hancock, N.; Dahm, K.; Guerra, K.; Mayer, X.; Wait, A.; Heil, D. An Integrated Framework for the Treatment and Management of Produced Water. Research Partnership to Secure Energy for America: RPSEA. 2011. Available online: http://www.rpsea.org/0712212/ (accessed on 24 May 2023).
- Zhao, S.; Huang, G.; Cheng, G.; Wang, Y.; Fu, H. Hardness, COD and turbidity removals from produced water by electrocoagulation pretreatment prior to Reverse Osmosis membranes. Desalination 2014, 344, 454–462. [Google Scholar] [CrossRef]
- Li, C.; Deng, W.; Gao, C.; Xiang, X.; Feng, X.; Batchelor, B.; Li, Y. Membrane distillation coupled with a novel two-stage pretreatment process for petrochemical wastewater treatment and reuse. Sep. Purif. Technol. 2019, 224, 23–32. [Google Scholar] [CrossRef]
- Gregory, K.B.; Vidic, R.D.; Dzombak, D.A. Water management challenges associated with the production of shale gas by hydraulic fracturing. Elements 2011, 7, 181–186. [Google Scholar] [CrossRef]
- Kerr, R.A. Bursts Onto the Scene. Sci. Mag. 2010, 328, 1624–1626. [Google Scholar]
- Ikonnikova, S.A.; Male, F.; Scanlon, B.R.; Reedy, R.C.; McDaid, G. Projecting the Water Footprint Associated with Shale Resource Production: Eagle Ford Shale Case Study. Environ. Sci. Technol. 2017, 51, 14453–14461. [Google Scholar] [CrossRef]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef] [PubMed]
- Hedar, Y.; Budiyono. Pollution Impact and Alternative Treatment for Produced Water. E3S Web Conf. 2018, 31, 03004. [Google Scholar] [CrossRef]
- Abousnina, R.M.; Nghiem, L.D.; Bundschuh, J. Comparison between oily and coal seam gas produced water with respect to quantity, characteristics and treatment technologies: A review. Desalin. Water Treat. 2015, 54, 1793–1808. [Google Scholar] [CrossRef]
- Ottaviano, J.G.; Cai, J.; Murphy, R.S. Assessing the decontamination efficiency of a three-component flocculating system in the treatment of oilfield-produced water. Water Res. 2014, 52, 122–130. [Google Scholar] [CrossRef]
- Kondash, A.; Vengosh, A. Water Footprint of Hydraulic Fracturing. Environ. Sci. Technol. Lett. 2015, 2, 276–280. [Google Scholar] [CrossRef]
- Kondash, A.J.; Lauer, N.E.; Vengosh, A. The intensification of the water footprint of hydraulic fracturing. Sci. Adv. 2018, 5, eaax8764. [Google Scholar] [CrossRef] [PubMed]
- Vidic, R.D.; Brantley, S.L.; Vandenbossche, J.M.; Yoxtheimer, D.; Abad, J.D. Impact of shale gas development on regional water quality. Science 2013, 340, 1235009. [Google Scholar] [CrossRef]
- Mccabe, P.J. Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2012. [Google Scholar] [CrossRef]
- Dickhout, J.M.; Moreno, J.; Biesheuvel, P.M.; Boels, L.; Lammertink, R.G.H.; de Vos, W.M. Produced water treatment by membranes: A review from a colloidal perspective. J. Colloid Interface Sci. 2017, 487, 523–534. [Google Scholar] [CrossRef]
- Bagheri, M.; Roshandel, R.; Shayegan, J. Optimal selection of an integrated produced water treatment system in the upstream of oil industry. Process Saf. Environ. Prot. 2018, 117, 67–81. [Google Scholar] [CrossRef]
- Ntongha, O.; and Obire, O. Impact of Oil Field Wastewater from Santa Barbara Oil Rig Location on the Microbial Population of Santa Barbara River in Bayelsa State, Nigeria. Acta Sci. Microbiol. 2022, 45–51. [Google Scholar] [CrossRef]
- Barbot, E.; Vidic, N.S.; Gregory, K.B.; Vidic, R.D. Spatial and temporal correlation of water quality parameters of produced waters from Devonian-age shale following hydraulic fracturing. Environ. Sci. Technol. 2013, 47, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Murali Mohan, A.; Hartsock, A.; Bibby, K.J.; Hammack, R.W.; Vidic, R.D.; Gregory, K.B. Microbial community changes in hydraulic fracturing fluids and produced water from shale gas extraction. Environ. Sci. Technol. 2013, 47, 13141–13150. [Google Scholar] [CrossRef]
- Soeder, D.J.; Kappel, W.M. Water Resources and Natural Gas Production from the Marcellus Shale; US Department of the Interior: Reston, VA, USA, 2009.
- Martin, J.P.; Hill, D.G.; Lombardi, T.E. Fractured shale gas potential in New York. Northeast. Geol. Environ. Sci. 2004, 26, 57–78. [Google Scholar]
- Bakke, T.; Klungsøyr, J.; Sanni, S. Environmental impacts of produced water and drilling waste discharges from the Norwegian offshore petroleum industry. Mar. Environ. Res. 2013, 92, 154–169. [Google Scholar] [CrossRef]
- Gazali, A.K.; Alkali, A.N.; Mohammed, Y.; Djauro, Y.; Muhammed, D.D.; Kodomi, M. Environmental Impact of Produced Water and Driiling Waste Discharges from the Niger Delta Petroleum Industry. IOSR J. Eng. 2017, 7, 22–29. [Google Scholar] [CrossRef]
- Robinson, D. Oil and gas: Water treatment in oil and gas production—Does it matter? Filtr. Sep. 2010, 47, 14–18. [Google Scholar] [CrossRef]
- Baza, J.; Chard, S. Produced water report: Regulations, current practices, and research needs. In Proceedings of the WEFTEC 2019-92nd Annual Water Environment Federation’s Technical Exhibition and Conference, Chicago, IL, USA, 21–25 September 2019; pp. 2483–2491. [Google Scholar]
- Veil, J. US Produced Water Volumes and Management Practices in 2012; Report Prepared for the Groundwater Protection Council; Groundwater Protection Council: Oklahoma City, OK, USA, 2015; p. 119. [Google Scholar]
- Veil, J. Produced Water Volumes and Management Practices for 2017; Ground Water Protection Council: Oklahoma City, OK, USA, 2020; Available online: https://www.gwref.net/ (accessed on 14 August 2023).
- Costa, T.C.; Hendges, L.T.; Temochko, B.; Mazur, L.P.; Marinho, B.A.; Weschenfelder, S.E.; Florido, P.L.; da Silva, A.; Ulson de Souza, A.A.; Guelli Ulson de Souza, S.M.A. Evaluation of the technical and environmental feasibility of adsorption process to remove water soluble organics from produced water: A review. J. Pet. Sci. Eng. 2022, 208, 109360. [Google Scholar] [CrossRef]
- PJSC Rosneft Oil Company. PJSC Rosneft Oil Company Annual Report; PJSC Rosneft Oil Company: Moscow, Russia, 2018; Available online: https://www.rosneft.com/upload/site2/document_file/a_report_2018_eng.pdf (accessed on 14 August 2023).
- ESCWA. ESCWA Water Development Report 6: The Water, Energy and Food Security Nexus in the Arab Region; ESCWA: Beirut, Lebanon, 2015. [Google Scholar]
- Petroleum Development Oman (PDO). Sustainability Report; Petroleum Development Oman (PDO): Muscat, Oman, 2018; Available online: https://www.pdo.co.om/en/news/publications/Publications%20Doc%20Library/PDO%20SR%202018_EA.pdf (accessed on 12 August 2023).
- Petrobras. Sustentabilidade; Petrobras: Rio de Janeiro, Brazil, 2017; Available online: https://sustentabilidad.ypf.com/assets/docs/en/YPF-Sustainability-report-2017.pdf (accessed on 12 August 2023).
- Petrobras. Sustentabilidade; Petrobras: Rio de Janeiro, Brazil, 2018; Available online: https://api.mziq.com/mzfilemanager/v2/d/25fdf098-34f5-4608-b7fa-17d60b2de47d/fd23adf5-6801-fcb1-c0fd-2c699bed5887?origin=1 (accessed on 12 August 2023).
- Saudi Aramco. This Is Energy, This Is Aramco. 2019. Available online: https://www.aramco.com/-/media/publications/corporate-reports/saudi-aramco-ara-2019-english.pdf (accessed on 12 May 2023).
- Cruz, H.; Law, Y.Y.; Guest, J.S.; Rabaey, K.; Batstone, D.; Laycock, B.; Verstraete, W.; Pikaar, I. Mainstream Ammonium Recovery to Advance Sustainable Urban Wastewater Management. Environ. Sci. Technol. 2019, 53, 11066–11079. [Google Scholar] [CrossRef]
- Gerritsen, M.G.; Durlofsky, L.J. Modeling fluid flow in oil reservoirs. Annu. Rev. Fluid Mech. 2005, 37, 211–238. [Google Scholar] [CrossRef]
- White, C.M.; Mungal, M.G. Mechanics and prediction of turbulent drag reduction with polymer additives. Annu. Rev. Fluid Mech. 2008, 40, 235–256. [Google Scholar] [CrossRef]
- Barati, R.; Liang, J.T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Domen, J.K.; Camarillo, M.K.; Sandelin, W.L.; Borglin, S. Physical, chemical, and biological characteristics of compounds used in hydraulic fracturing. J. Hazard. Mater. 2014, 275, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, G.A.; Blotevogel, J.; Stewart, P.S.; Borch, T. Biocides in hydraulic fracturing fluids: A critical review of their usage, mobility, degradation, and toxicity. Environ. Sci. Technol. 2015, 49, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Fu, Q. Ensuring better well stimulation in unconventional oil and gas formations by optimizing surfactant additives. In Proceedings of the Society of Petroleum Engineers Western Regional Meeting, Bakersfield, CA, USA, 21–23 March 2012; pp. 949–955. [Google Scholar] [CrossRef]
- Lester, Y.; Ferrer, I.; Thurman, E.M.; Sitterley, K.A.; Korak, J.A.; Aiken, G.; Linden, K.G. Characterization of hydraulic fracturing flowback water in Colorado: Implications for water treatment. Sci. Total Environ. 2015, 512–513, 637–644. [Google Scholar] [CrossRef]
- Nicot, J.P.; Scanlon, B.R. Water use for shale-gas production in Texas, U.S. Environ. Sci. Technol. 2012, 46, 3580–3586. [Google Scholar] [CrossRef] [PubMed]
- Raimi, D. Comment on “The intensification of the water footprint of hydraulic fracturing”. Sci. Adv. 2020, 6, aav2110. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Nicot, J.P. Comparison of water use for hydraulic fracturing for unconventional oil and gas versus conventional oil. Environ. Sci. Technol. 2014, 48, 12386–12393. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, T.J.; Varela, B.A.; Haines, S.S.; Engle, M.A. Hydraulic fracturing water use variability in the United States and potential environmental implications. Water Resour. Res. 2015, 51, 5839–5845. [Google Scholar] [CrossRef]
- Gallegos, T.; Varela, B. Trends in Hydraulic Fracturing Distributions and Treatment Fluids, Additives, Proppants, and Water Volumes Applied to Wells Drilled in the United States from 1947; USGS: Reston, VA, USA, 2014. Available online: https://pubs.usgs.gov/sir/2014/5131/ (accessed on 24 August 2023).
- Walker, E.L.; Anderson, A.M.; Read, L.K.; Hogue, T.S. Water Use for Hydraulic Fracturing of Oil and Gas in the South Platte River Basin, Colorado. J. Am. Water Resour. Assoc. 2017, 53, 839–853. [Google Scholar] [CrossRef]
- Lin, Z.; Lin, T.; Lim, S.; Hove, M.; Schuh, W. Impacts of Bakken Shale Oil Development on Regional Water Uses and Supply. JAWRA J. Am. Water Resour. Assoc. 2017, 54, 225–239. [Google Scholar] [CrossRef]
- Rosa, L.; Rulli, M.C.; Davis, K.F.; D’Odorico, P. The Water-Energy Nexus of Hydraulic Fracturing: A Global Hydrologic Analysis for Shale Oil and Gas Extraction. Earth’s Future 2017, 6, 745–756. [Google Scholar] [CrossRef]
- Freyman, M. Hydraulic Fracturing & Water Stress: Water Demand by the Numbers; Ceres: Boston, MA, USA, 2014; p. 85. [Google Scholar]
- Reig, P.; Luo, T.; Proctor, J.N. Global Shale Gas Development: Water Availability and Business Risks; World Resources Institute: Washington, DC, USA, 2014; pp. 1–80. Available online: https://www.wri.org/publication/global-shale-gas-development-water-availability-business-risks (accessed on 12 May 2023).
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J.; Yaghi, O.M. Practical water production from desert air. Sci. Adv. 2018, 4, eaat3198. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Male, F.; Walsh, M. Water Issues Related to Transitioning from Conventional to Unconventional Oil Production in the Permian Basin. Environ. Sci. Technol. 2017, 51, 10903–10912. [Google Scholar] [CrossRef]
- Fasola, S.L.; Brudzinski, M.R.; Skoumal, R.J.; Langenkamp, T.; Currie, B.S.; Smart, K.J. Hydraulic Fracture Injection Strategy Influences the Probability of Earthquakes in the Eagle Ford Shale Play of South Texas. Geophys. Res. Lett. 2019, 46, 12958–12967. [Google Scholar] [CrossRef]
- Skoumal, R.J.; Ries, R.; Brudzinski, M.R.; Barbour, A.J.; Currie, B.S. Earthquakes Induced by Hydraulic Fracturing Are Pervasive in Oklahoma. J. Geophys. Res. Solid Earth 2018, 123, 10918–10935. [Google Scholar] [CrossRef]
- Murray, K.E. State-scale perspective on water use and production associated with oil and gas operations, Oklahoma, U.S. Environ. Sci. Technol. 2013, 47, 4918–4925. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, B.R.; Weingarten, M.B.; Murray, K.E.; Reedy, R.C. Managing basin-scale fluid budgets to reduce injection-induced seismicity from the recent U.S. Shale oil revolution. Seismol. Res. Lett. 2019, 90, 171–182. [Google Scholar] [CrossRef]
- Ferguson, G. Deep injection of wastewater in the western Canada sedimentary basin. Groundwater 2015, 53, 187–194. [Google Scholar] [CrossRef]
- Webb, R.; Zodrow, K.R. Disposal of Water for Hydraulic Fracturing: Case Study on the U.S. In Regulating Water Security in Unconventional Oil and Gas; Buono, R., López Gunn, E., McKay, J., Staddon, C., Eds.; Water Security in a New World; Springer: Cham, Switzerland, 2020; p. 11. [Google Scholar] [CrossRef]
- Frohlich, C. Two-year survey comparing earthquake activity and injection-well locations in the Barnett Shale, Texas. Proc. Natl. Acad. Sci. USA 2012, 109, 13934–13938. [Google Scholar] [CrossRef] [PubMed]
- Frohlich, C.; De Shon, H.; Stump, B.; Hayward, C.; Hornbach, M.; Walter, J.I. A historical review of induced Earthquakes in Texas. Seismol. Res. Lett. 2016, 87, 1022–1038. [Google Scholar] [CrossRef]
- Weingarten, M.; Ge, S.; Godt, J.W.; Bekins, B.A.; Rubinstein, J.L. High-rate injection is associated with the increase in U.S. mid-continent seismicity. Science 2015, 348, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, G.T.; Dorman, F.; Westland, J.L.; Yoxtheimer, D.; Grieve, P.; Sowers, T.; Humston-Fulmer, E.; Brantley, S.L. Evaluating a groundwater supply contamination incident attributed to Marcellus Shale gas development. Proc. Natl. Acad. Sci. USA 2015, 112, 6325–6330. [Google Scholar] [CrossRef] [PubMed]
- Jasechko, S.; Perrone, D. Hydraulic fracturing near domestic groundwater wells. Proc. Natl. Acad. Sci. USA 2017, 114, 13138–13143. [Google Scholar] [CrossRef]
- Rahm, B.G.; Riha, S.J. Evolving Shale Gas Management: Water Resource Risks, Impacts, and Lessons Learned. Environ. Sci. Process Impacts 2014, 16, 1400–1412. [Google Scholar] [CrossRef]
- Patterson, L.A.; Konschnik, K.E.; Wiseman, H.; Fargione, J.; Maloney, K.O.; Kiesecker, J.; Nicot, J.P.; Baruch-Mordo, S.; Entrekin, S.; Trainor, A.; et al. Unconventional Oil and Gas Spills: Risks, Mitigation Priorities, and State Reporting Requirements. Environ. Sci. Technol. 2017, 51, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Brantley, S.L. Drinking Water While Fracking: Now and in the Future. Ground Water 2015, 53, 21–23. [Google Scholar]
- Faksness, L.G.; Grini, P.G.; Daling, P.S. Partitioning of semi-soluble organic compounds between the water phase and oil droplets in produced water. Mar. Pollut. Bull. 2004, 48, 731–742. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Z.; Lee, K. A Risk Assessment Model for Produced Water Discharge from Offshore Petroleum Platforms: Development and Validation. Mar. Pollut. Bull. 2008, 56, 1890–1897. [Google Scholar] [CrossRef]
- Hermosilla, D.; Merayo, N.; Gascó, A.; Blanco, Á. The Application of Advanced Oxidation Technologies to the Treatment of Effluents from the Pulp and Paper Industry: A Review. Environ. Sci. Pollut. Res. Int. 2015, 22, 168–191. [Google Scholar] [CrossRef]
- Dores, R.; Hussain, A.; Katebah, M.A.; Adham, S. Using Advanced Water Treatment Technologies to Treat Produced Water from the Petroleum Industry. In Proceedings of the Paper presented at the SPE International Production and Operations Conference & Exhibition, Doha, Qatar, 14–16 May 2012. [Google Scholar] [CrossRef]
- Bader, M. Seawater versus produced water in oil-fields water injection operations. Desalination 2006, 208, 159–168. [Google Scholar] [CrossRef]
- Oetjen, K.; Giddings CG, S.; McLaughlin, M.; Nell, M.; Blotevogel, J.; Helbling, D.E.; Mueller, D.; Higgins, C.P. Emerging analytical methods for the characterization and quantification of organic contaminants in flowback and produced water. Trends Environ. Anal. Chem. 2017, 15, 12–23. [Google Scholar] [CrossRef]
- Cho, H.; Jang, Y.; Koo, J.; Choi, Y.; Lee, S.; Sohn, J. Effect of pretreatment on fouling propensity of shale gas wastewater in membrane distillation process. Desalin. Water Treat. 2017, 57, 24566–24573. [Google Scholar] [CrossRef]
- He, C.; Vidic, R.D. Application of microfiltration for the treatment of Marcellus Shale flowback water: Influence of floc breakage on membrane fouling. J. Membr. Sci. 2016, 510, 348–354. [Google Scholar] [CrossRef]
- Blondes, M.S.; Gans, K.D.; Rowan, E.L.; Thordsen, J.J.; Reidy, M.E.; Engle, M.A.; Kharaka, Y.K.; Thomas, B.U.S. Geological Survey National Produced Waters Geochemical Database v2.2 Documentation; USGS: Reston, VA, USA, 2016; Volume 3, p. 28.
- Palacios, V. Baseline Groundwater Quality Testing Needs in the Eagle Ford Shale Region. Master’s Thesis, Duke University, Durham, NC, USA, 2012. Available online: http://hdl.handle.net/10161/5370 (accessed on 20 May 2023).
- Strong, L.C.; Gould, T.; Kasinkas, L.; Sadowsky, M.J.; Aksan, A.; Wackett, L.P. Biodegradation in Waters from Hydraulic Fracturing: Chemistry, Microbiology, and Engineering. J. Environ. Eng. 2014, 140, B4013001. [Google Scholar] [CrossRef]
- Hayes, T.; Severin, B.F. Barnett and Appalachian Shale Water Management and Reuse Technologies RPSEA Report No 08122-05; Project Report by Gas Technology Institute for Research Partnership to Secure Energy for America (RPSEA); RPSEA: Houston, TX, USA, 2012; Volume 1700, pp. 1–125. [Google Scholar]
- Zhang, H.; Xiong, Z.; Ji, F.; Lai, B.; Yang, P. Pretreatment of shale gas drilling flowback fluid (SGDF) by the microscale Fe0/persulfate/O3 process (mFe0/PS/O3). Chemosphere 2017, 176, 192–201. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Z.; Nghiem, L.D.; Li, X.M.; Xie, M.; Zhao, B.; Zhang, M.; Song, J.; He, T. Treatment of shale gas drilling flowback fluids (SGDFs) by forward osmosis: Membrane fouling and mitigation. Desalination 2015, 366, 113–120. [Google Scholar] [CrossRef]
- Freedman, D.E.; Riley, S.M.; Jones, Z.L.; Rosenblum, J.S.; Sharp, J.O.; Spear, J.R.; Cath, T.Y. Biologically active filtration for fracturing flowback and produced water treatment. J. Water Process Eng. 2017, 18, 29–40. [Google Scholar] [CrossRef]
- Greenlee, L.F.; Lawler, D.F.; Freeman, B.D.; Marrot, B.; Moulin, P. Reverse Osmosis Desalination: Water Sources, Technology, and Today’s Challenges. Water Res. 2009, 43, 2317–2348. [Google Scholar] [CrossRef] [PubMed]
- Orem, W.; Tatu, C.; Varonka, M.; Lerch, H.; Bates, A.; Engle, M.; Crosby, L.; McIntosh, J. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale. Int. J. Coal Geol. 2014, 126, 20–31. [Google Scholar] [CrossRef]
- Kong, F.; Xin, J.F.; Wang, H.M.; Liu, X.N.; Wang, X.M.; Wen, X.; Chen, C.M.; Xie, Y.F. Application of coagulation-UF hybrid process for shale gas fracturing flowback water recycling: Performance and fouling analysis. J. Membr. Sci. 2017, 524, 460–469. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.; Chen, M.; Ma, L.; Wang, H.; Wang, S. Wet air oxidation of fracturing flowback fluids over promoted bimetallic Cu-Cr catalyst. Catal. Commun. 2017, 90, 60–64. [Google Scholar] [CrossRef]
- Coday, B.D.; Almaraz, N.; Cath, T.Y. Forward osmosis desalination of oil and gas wastewater: Impacts of membrane selection and operating conditions on process performance. J. Membr. Sci. 2015, 488, 40–55. [Google Scholar] [CrossRef]
- Acharya, H.R.; Henderson, C.; Wang, H. Cost Effective Recovery of Low-TDS Frac Flowback Water for Re-Use; General Electric Co.: Boston, MA, USA, 2011; pp. 1–100. [Google Scholar]
- Khan, N.A.; Engle, M.; Dungan, B.; Holguin, F.O.; Xu, P.; Carroll, K.C. Volatile-organic molecular characterization of shale-oil produced water from the Permian Basin. Chemosphere 2016, 148, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Coday, B.D.; Cath, T.Y. Forward Osmosis: Novel Desalination of Produced Water and Fracturing Flowback. J. Am. Water Works Assoc. 2014, 106, E55–E66. [Google Scholar] [CrossRef]
- Cho, H.; Choi, Y.; Lee, S. Effect of pretreatment and operating conditions on the performance of membrane distillation for the treatment of shale gas wastewater. Desalination 2018, 437, 195–209. [Google Scholar] [CrossRef]
- Chorghe, D.; Sari, M.A.; Chellam, S. Boron removal from hydraulic fracturing wastewater by aluminum and iron coagulation: Mechanisms and limitations. Water Res. 2017, 126, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, N.; Chilkoor, G.; Wilder, J.; Ren, Z.J.; Gadhamshetty, V. Comparative performances of microbial capacitive deionization cell and microbial fuel cell fed with produced water from the Bakken shale. Bioelectrochemistry 2018, 121, 56–64. [Google Scholar] [CrossRef]
- Monzon, O.; Yang, Y.; Kim, J.; Heldenbrand, A.; Li, Q.; Alvarez, P.J. Microbial fuel cell fed by Barnett Shale produced water: Power production by hypersaline autochthonous bacteria and coupling to a desalination unit. Biochem. Eng. J. 2017, 117, 87–91. [Google Scholar] [CrossRef]
- Rosenblum, J.S.; Sitterley, K.A.; Thurman, E.M.; Ferrer, I.; Linden, K.G. Hydraulic fracturing wastewater treatment by coagulation-adsorption for removal of organic compounds and turbidity. J. Environ. Chem. Eng. 2016, 4, 1978–1984. [Google Scholar] [CrossRef]
- Li, X.M.; Zhao, B.; Wang, Z.; Xie, M.; Song, J.; Nghiem, L.D.; He, T.; Yang, C.; Li, C.; Chen, G. Water reclamation from shale gas drilling flow-back fluid using a novel forward osmosis-vacuum membrane distillation hybrid system. Water Sci. Technol. 2014, 69, 1036–1044. [Google Scholar] [CrossRef]
- Shih, J.S.; Saiers, J.E.; Anisfeld, S.C.; Chu, Z.; Muehlenbachs, L.A.; Olmstead, S.M. Characterization and Analysis of Liquid Waste from Marcellus Shale Gas Development. Environ. Sci. Technol. 2015, 49, 9557–9565. [Google Scholar] [CrossRef] [PubMed]
- Thiel, G.P.; Lienhard, V.J.H. Treating produced water from hydraulic fracturing: Composition effects on scale formation and desalination system selection. Desalination 2014, 346, 54–69. [Google Scholar] [CrossRef]
- Miller, D.J.; Huang, X.; Li, H.; Kasemset, S.; Lee, A.; Agnihotri, D.; Hayes, T.; Paul, D.R.; Freeman, B.D. Fouling-resistant membranes for the treatment of flowback water from hydraulic shale fracturing: A pilot study. J. Membr. Sci. 2013, 437, 265–275. [Google Scholar] [CrossRef]
- Hawari, A.H.; Alkhatib, A.M.; Hafiz, M.; Das, P. A Novel Electrocoagulation Electrode Configuration for the Removal of Total Organic Carbon from Primary Treated Municipal Wastewater. Environ. Sci. Pollut. Res. Int. 2020, 27, 23888–23898. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fan, H.; Xie, H.; Huang, Z. Experimental study of treatment processes for shale gas fracturing flowback fluid in the eastern Sichuan Basin. Desalin. Water Treat. 2016, 57, 24299–24312. [Google Scholar] [CrossRef]
- Regnery, J.; Coday, B.D.; Riley, S.M.; Cath, T.Y. Solid-phase extraction followed by gas chromatography-mass spectrometry for the quantitative analysis of semi-volatile hydrocarbons in hydraulic fracturing wastewaters. Anal. Methods 2016, 8, 2058–2068. [Google Scholar] [CrossRef]
- Carrero-Parreño, A.; Reyes-Labarta, J.A.; Salcedo-Díaz, R.; Ruiz-Femenia, R.; Onishi, V.C.; Caballero, J.A.; Grossmann, I.E. Holistic Planning Model for Sustainable Water Management in the Shale Gas Industry. Ind. Eng. Chem. Res. 2018, 57, 13131–13143. [Google Scholar] [CrossRef]
- Xiong, B.; Zydney, A.L.; Kumar, M. Fouling of microfiltration membranes by flowback and produced waters from the Marcellus shale gas play. Water Res. 2016, 99, 162–170. [Google Scholar] [CrossRef]
- Sari, M.A.; Chellam, S. Mechanisms of boron removal from hydraulic fracturing wastewater by aluminum electrocoagulation. J. Colloid Interface Sci. 2015, 458, 103–111. [Google Scholar] [CrossRef]
- Ali, A.; Jacobsen, J.H.; Jensen, H.C.; Christensen, M.L.; Quist-Jensen, C.A. Treatment of Wastewater Solutions from Anodizing Industry by Membrane Distillation and Membrane Crystallization. Appl. Sci. 2019, 9, 287. [Google Scholar] [CrossRef]
- Lauer, N.E.; Harkness, J.S.; Vengosh, A. Brine Spills Associated with Unconventional Oil Development in North Dakota. Environ. Sci. Technol. 2016, 50, 5389–5397. [Google Scholar] [CrossRef]
- Al-Rekabi, S.W.; Qiang, H.; Qiang, W. Improvements in Wastewater Treatment Technology. Pakistan J. Nutr. 2007, 6, 104–110. [Google Scholar]
- Chang, H.; Liu, T.; He, Q.; Li, D.; Crittenden, J.; Liu, B. Removal of calcium and magnesium ions from shale gas flowback water by chemically activated zeolite. Water Sci. Technol. 2017, 76, 575–583. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Hong, S. Recovery of water and minerals from shale gas produced water by membrane distillation crystallization. Water Res. 2018, 129, 447–459. [Google Scholar] [CrossRef]
- Bell, E.A.; Poynor, T.E.; Newhart, K.B.; Regnery, J.; Coday, B.D.; Cath, T.Y. Produced water treatment using forward osmosis membranes: Evaluation of extended-time performance and fouling. J. Membr. Sci. 2017, 525, 77–88. [Google Scholar] [CrossRef]
- Abualfaraj, N.; Gurian, P.L.; Olson, M.S. Characterization of Marcellus shale flowback water. Environ. Eng. Sci. 2014, 31, 514–524. [Google Scholar] [CrossRef]
- Rosenblum, J.; Thurman, E.M.; Ferrer, I.; Aiken, G.; Linden, K.G. Organic Chemical Characterization and Mass Balance of a Hydraulically Fractured Well: From Fracturing Fluid to Produced Water over 405 Days. Environ. Sci. Technol. 2017, 51, 14006–14015. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; Thomas He, Y. Evolution of water chemistry during Marcellus Shale gas development: A case study in West Virginia. Chemosphere 2017, 134, 224–231. [Google Scholar] [CrossRef]
- Masooleh, M.S.; Bazgir, S.; Tamizifar, M.; Nemati, A. Adsorption of Petroleum Hydrocarbons on Organoclay. J. Appl. Chem. Res. 2010, 4, 19–23. [Google Scholar]
- Barry, E.; Burns, R.; Chen, W.; De Hoe, G.X.; De Oca, J.M.M.; de Pablo, J.J.; Dombrowski, J.; Elam, J.W.; Felts, A.M.; Galli, G.; et al. Advanced Materials for Energy-Water Systems: The Central Role of Water/Solid Interfaces in Adsorption, Reactivity, and Transport. Chem. Rev. 2021, 121, 9450–9501. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Zhong, Q.Z.; Li, J.; Lin, Z.; Cui, J.; Caruso, F.; Hao, J. Metal Ion-Directed Functional Metal-Phenolic Materials. Chem. Rev. 2022, 122, 11432–11473. [Google Scholar] [CrossRef]
- Dawoud, H.D.; Saleem, H.; Alnuaimi, N.A.; Zaidi, S.J. Characterization and treatment technologies applied for produced water in Qatar. Water 2021, 13, 3573. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, H.; Li, Y.; Xu, H.; Pan, Z.; Dai, P.; Wang, H.; Yang, Q. A review of treatment technologies for produced water in offshore oil and gas fields. Sci. Total Environ. 2021, 775, 145485. [Google Scholar] [CrossRef]
- Majdalani, J. On the generalized Beltramian motion of the bidirectional vortex in a conical cyclone. Phys. Fluids 2022, 34, 36604. [Google Scholar] [CrossRef]
- Lefebvre, O.; Moletta, R. Treatment of Organic Pollution in Industrial Saline Wastewater: A Literature Review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Rawindran, H.; Krishnan, S.; Sinnathambi, C.M. A Review on overboard CEOR discharged produced water treatment and remediation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 206, 12086. [Google Scholar] [CrossRef]
- Schuetz, S.; Mayer, G.; Bierdel, M.; Piesche, M. Investigations on the flow and separation behaviour of hydrocyclones using computational fluid dynamics. Int. J. Miner. Process. 2004, 73, 229–237. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Evgenidou, E.; Lambropoulou, D.; Konstantinou, I. A Review on Advanced Oxidation Processes for the Removal of Taste and Odor Compounds from Aqueous Media. Water Res. 2014, 53, 215–234. [Google Scholar] [CrossRef]
- Warsinger, D.M.; Mistry, K.H.; Nayar, K.G.; Chung, H.W.; Lienhard, J.H. Entropy generation of desalination powered by variable temperature waste heat. Entropy 2015, 17, 7530–7566. [Google Scholar] [CrossRef]
- Ariga, K.; Lee, M.V.; Mori, T.; Yu, X.-Y.; Hill, J.P. Two-dimensional nanoarchitectonics based on self-assembly. Adv. Colloid Interface Sci. 2010, 154, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Khader, E.H.; Mohammed, T.J.; Mirghaffari, N.; Salman, A.D.; Juzsakova, T.; Abdullah, T.A. Removal of organic pollutants from produced water by batch adsorption treatment. Clean Technol. Environ. Policy 2020, 24, 713–720. [Google Scholar] [CrossRef]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of adsorbents and recovery of heavy metals: A review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
- Gao, Q.; Zhu, H.; Luo, W.-J.; Wang, S.; Zhou, C.G. Preparation, characterization, and adsorption evaluation of chitosan-functionalized mesoporous composites. Microporous Mesoporous Mater. 2014, 193, 15–26. [Google Scholar] [CrossRef]
- Dabrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef] [PubMed]
- Puri, C.; Sumana, G. Highly effective adsorption of crystal violet dye from contaminated water using graphene oxide intercalated montmorillonite nanocomposite. Appl. Clay Sci. 2018, 166, 102–112. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Pan, L.; Zhang, X.; Zou, J.J. Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: A review. Nanoscale 2020, 12, 4790–4815. [Google Scholar] [CrossRef] [PubMed]
- Eldin, M.S.M.; Aggour, Y.A.; Elaassar, M.R.; Beghet, G.E.; Atta, R.R. Development of nano-crosslinked polyacrylonitrile ions exchanger particles for dye removal: Kinetic, isotherm, and thermodynamic studies. Desalin. Water Treat 2020, 175, 24911. [Google Scholar]
- White, J.E.; Catallo, W.J.; Legendre, B.L. Biomass pyrolysis kinetics: A comparative critical review with relevant agricultural residue case studies. J. Anal. Appl. Pyrolysis 2011, 91, 1–33. [Google Scholar] [CrossRef]
- Brandani, S. Kinetics of Liquid Phase Batch Adsorption Experiments. Adsorption 2021, 27, 353–368. [Google Scholar] [CrossRef]
- Eow, J.S.; Ghadiri, M. Electrostatic Enhancement of Coalescence of Water Droplets in Oil: A Review of the Technology. Chem. Eng. J. 2002, 85, 357–368. [Google Scholar] [CrossRef]
- Röhrs, J.; Dagestad, K.-F.; Asbjørnsen, H.; Nordam, T.; Skancke, J.; Jones, C.E.; Brekke, C. The Effect of Vertical Mixing on the Horizontal Drift of Oil Spills. Ocean Sci. 2018, 14, 1581–1601. [Google Scholar] [CrossRef]
- Salem, F.; Thiemann, T. Produced Water from Oil and Gas Exploration—Problems, Solutions and Opportunities. J. Water Resour. Prot. 2022, 14, 142–185. [Google Scholar] [CrossRef]
- López-Vazquez, C.M.; Fall, C. Improvement of a gravity oil separator using a designed experiment. Water Air Soil Pollut. 2004, 157, 33–52. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Oil and grease removal from wastewaters: Sorption treatment as an alternative to state-of-the-art technologies. A critical review. Chem. Eng. J. 2016, 297, 229–255. [Google Scholar] [CrossRef]
- Wang, J.; Tanuwidjaja, D.; Bhattacharjee, S.; Edalat, A.; Jassby, D.; Hoek, E.M.V. Produced water desalination via pervaporative distillation. Water 2020, 12, 3560. [Google Scholar] [CrossRef]
- Forero, J.-E.; Ortíz, O.-P.; Nariño, F.-A.; Díaz, J.; Peña, H. Design and development of a high efficiency tank for crude oil dehydration (I). CT&F-Cienc. Tecnología Futuro 2008, 3, 185–199. [Google Scholar]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil–water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef]
- Pal, M.S.; Bhatia, M. Current Status, Topographical Constraints, and Implementation Strategy of Municipal Solid Waste in India: A Review. Arab. J. Geosci. 2022, 15, 1176. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Q.; Ma, H.; Huang, P.; Li, J.; Kikuchi, T. Effect of micro-bubbles on coagulation flotation process of dyeing wastewater. Sep. Purif. Technol. 2010, 71, 337–346. [Google Scholar] [CrossRef]
- Hocking, M.B. Handbook of Chemical Technology and Pollution Control; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Piccioli, M.; Aanesen, S.V.; Zhao, H.; Dudek, M.; Øye, G. Gas Flotation of Petroleum Produced Water: A Review on Status, Fundamental Aspects, and Perspectives. Energy Fuels 2020, 34, 15579–15592. [Google Scholar] [CrossRef]
- Ayoub, M. Performance Evaluation of an Oily Industrial Wastewater Treatment System Using the Application of Activated Sludge Model No.3. Water Environ. J. 2023. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Barani, M.; Bazgir, S.; Hosseini, M.K.; Hosseini, P.K. Eco-facile application of electrospun nanofibers to the oil-water emulsion separation via coalescing filtration in pilot-scale and beyond. Process Saf. Environ. Prot. 2021, 148, 342–357. [Google Scholar] [CrossRef]
- da Silva Almeida, F.B.P.; Esquerre, K.P.S.O.R.; Soletti, J.I.; De Farias Silva, C.E. Coalescence process to treat produced water: An updated overview and environmental outlook. Environ. Sci. Pollut. Res. 2019, 26, 28668–28688. [Google Scholar] [CrossRef]
- Mautner, A. Nanocellulose water treatment membranes and filters: A review. Polym. Int. 2020, 69, 741–751. [Google Scholar] [CrossRef]
- Bello, O.S.; Bello, I.A.; Adegoke, K.A. Adsorption of dyes using different types of sand: A review. S. Afr. J. Chem. 2013, 66, 117–129. [Google Scholar]
- Van der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Progress 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat transfer fluids for concentrating solar power systems—A review. Appl. Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Abuhasel, K.; Kchaou, M.; Alquraish, M.; Munusamy, Y.; Jeng, Y.T. Oily Wastewater Treatment: Overview of Conventional and Modern Methods, Challenges, and Future Opportunities. Water 2021, 13, 980. [Google Scholar] [CrossRef]
- Bhargava, A. Physico-chemical wastewater treatment technologies: An overview. Int. J. Sci. Res. Educ. 2016, 4, 5308–5319. [Google Scholar]
- Adetunji, A.I.; Olaniran, A.O. Treatment of Industrial Oily Wastewater by Advanced Technologies: A Review. Appl. Water Sci. 2021, 11, 98. [Google Scholar] [CrossRef]
- Santos, E.V.; Bezerra Rocha, J.H.; Araújo, D.M.; Moura, D.C.; Martínez-Huitle, C.A. Decontamination of Produced Water Containing Petroleum Hydrocarbons by Electrochemical Methods: A Minireview. Environ. Sci. Pollut. Res. 2014, 21, 8432–8441. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef]
- Abdulquadir, M.; Al-Ghouti, M.A.; Das, P.; Abu-Dieyeh, M.H.; Ahmed, T.; Aljabri, H. Potential Application of Microalgae in Produced Water Treatment. Desalin. Water Treat. 2018, 135, 47–58. [Google Scholar]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Hethnawi, A.; Mosleh, A.; Nassar, N.N. Nanoparticles for Cleaning up Oil Sands Process-Affected Water. In Nanoparticles: An Emerging Technology for Oil Production and Processing Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 445–496. [Google Scholar]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2012, 9, 157–177. [Google Scholar] [CrossRef]
- Wu, W.; Huang, Z.-H.; Lim, T.-T. Recent development of mixed metal oxide anodes for electrochemical oxidation of organic pollutants in water. Appl. Catal. A Gen. 2014, 480, 58–78. [Google Scholar] [CrossRef]
- Sirés, I.; Brillas, E.; Oturan, M.A.; Rodrigo, M.A.; Panizza, M. Electrochemical advanced oxidation processes: Today and tomorrow. A review. Environ. Sci. Pollut. Res. 2014, 21, 8336–8367. [Google Scholar] [CrossRef]
- Sehar, T.; Sheikh, G.G.; Zargar, M.Y.; Baba, Z.A. Identification and Screening of Earthworm Species from Various Temperate Areas of Kashmir Valley for Vermicomposting. Adv. Recycl. Waste Manag. 2016, 1, 102. [Google Scholar]
- Habieeb, A.R.; Kabeel, A.E.; Sultan, G.I.; Abdelsalam, M.M. Advancements in Water Desalination Through Artificial Intelligence: A Comprehensive Review of AI-Based Methods for Reverse Osmosis Membrane Processes. Water Conserv. Sci. Eng. 2023, 8, 53. [Google Scholar] [CrossRef]
- Davis, T.A.; Grebenyuk, V.; Grebenyuk, O. Electromembrane processes. In Membrane Technology: In the Chemical Industry; Willey: Hoboken, NJ, USA, 2001; pp. 222–267. [Google Scholar]
- Hayes, T.; Arthur, D. Overview of emerging produced water treatment technologies. In Proceedings of the 11th Annual International Petroleum Environmental Conference, Albuquerque, NM, USA, 11–15 October 2004; p. 201512. [Google Scholar]
- Sosa-Fernandez, P.A.; Post, J.W.; Ramdlan, M.S.; Leermakers, F.A.M.; Bruning, H.; Rijnaarts, H.H.M. Improving the performance of polymer-flooding produced water electrodialysis through the application of pulsed electric field. Desalination 2020, 484, 114424. [Google Scholar] [CrossRef]
- Natarajan, S.; Bajaj, H.C.; Tayade, R.J. Recent advances based on the synergetic effect of adsorption for removal of dyes from wastewater using photocatalytic process. J. Environ. Sci. 2018, 65, 201–222. [Google Scholar] [CrossRef]
- Mezher, T.; Fath, H.; Abbas, Z.; Khaled, A. Techno-economic assessment and environmental impacts of desalination technologies. Desalination 2011, 266, 263–273. [Google Scholar] [CrossRef]
- Ge, J.; Zhang, Y.; Heo, Y.-J.; Park, S.-J. Advanced design and synthesis of composite photocatalysts for the remediation of wastewater: A review. Catalysts 2019, 9, 122. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Wang, N.; Zhao, Q.; Wang, P. Status of the treatment of produced water containing polymer in oilfields: A review. J. Environ. Chem. Eng. 2021, 9, 105303. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef] [PubMed]
- Mudhoo, A.; Bhatnagar, A.; Rantalankila, M.; Srivastava, V.; Sillanpää, M. Endosulfan removal through bioremediation, photocatalytic degradation, adsorption and membrane separation processes: A review. Chem. Eng. J. 2019, 360, 912–928. [Google Scholar] [CrossRef]
- Elsaid, K.; Kamil, M.; Sayed, E.T.; Abdelkareem, M.A.; Wilberforce, T.; Olabi, A. Environmental impact of desalination technologies: A review. Sci. Total Environ. 2020, 748, 141528. [Google Scholar] [CrossRef]
- Millar, G.J.; Lin, J.; Arshad, A.; Couperthwaite, S.J. Evaluation of electrocoagulation for the pre-treatment of coal seam water. J. Water Process Eng. 2014, 4, 166–178. [Google Scholar] [CrossRef]
- Hong, P.K.; Xiao, T. Treatment of oil spill water by ozonation and sand filtration. Chemosphere 2013, 91, 641–647. [Google Scholar] [CrossRef]
- Druskovic, M.; Vouk, D.; Posavcic, H.; Halkijevic, I.; Nad, K. The Application of Electrochemical Processes in Oily Wastewater Treatment: A Review. J. Environ. Sci. Health A Tox Hazard Subst Environ Eng. 2021, 56, 1373–1386. [Google Scholar] [CrossRef] [PubMed]
- Knapik, E. Biodemulsification combined with fixed bed biosorption for the recovery of crude oil from produced water. J. Water Process Eng. 2020, 38, 101614. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Krivoruchko, A.V.; Ivshina, I.B. Advanced Bioreactor Treatments of Hydrocarbon-Containing Wastewater. Applied Sciences 2020, 10, 831. [Google Scholar] [CrossRef]
- Pal, S.; Banat, F.; Almansoori, A.; Abu Haija, M. Review of technologies for biotreatment of refinery wastewaters: Progress, challenges, and future opportunities. Environ. Technol. Rev. 2016, 5, 12–38. [Google Scholar] [CrossRef]
- Mousa, I.E. Total petroleum hydrocarbon degradation by hybrid electrobiochemical reactor in oilfield produced water. Mar. Pollut. Bull. 2016, 109, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.; Zhang, Y.; Liu, G.; Ye, Z.; Chu, P.K. Treatment of heavy oil wastewater by a conventional activated sludge process coupled with an immobilized biological filter. Int. Biodeterior. Biodegrad. 2013, 84, 65–71. [Google Scholar] [CrossRef]
- Zolfaghari, M.; Drogui, P.; Blais, J.F. Removal of Macro-Pollutants in Oily Wastewater Obtained from Soil Remediation Plant Using Electro-Oxidation Process. Environ. Sci. Pollut. Res. Int. 2018, 25, 7748–7757. [Google Scholar] [CrossRef]
- Ezazi, M.; Quazi, M.M.; Taheri, H. Recent Studies of Membranes for Liquids Separation and Water Treatment. Membranes 2023, 13, 779. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Boyraz, E.; Maryska, J.; Kucerova, K. A Review on Membrane Technology and Chemical Surface Modification for the Oily Wastewater Treatment. Materials 2020, 13, 493. [Google Scholar] [CrossRef]
- Yang, X.; Wen, Y.; Li, Y.; Yan, L.; Tang, C.Y.; Ma, J.; Darling, S.B.; Shao, L. Engineering In Situ Catalytic Cleaning Membrane Via Prebiotic-Chemistry-Inspired Mineralization. Adv. Mater. 2023, 2306626. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Yang, X.; Martinson, A.B.F.; Elam, J.W.; Shao, L.; Darling, S.B. Water treatment based on atomically engineered materials: Atomic layer deposition and beyond. Matter 2021, 4, 3515–3548. [Google Scholar] [CrossRef]
- Yang, X.; Sun, P.; Zhang, H.; Xia, Z.; Waldman, R.Z.; Mane, A.U.; Elam, J.W.; Shao, L.; Darling, S.B. Polyphenol-Sensitized Atomic Layer Deposition for Membrane Interface Hydrophilization. Adv. Funct. Mater. 2020, 30, 1910062. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization—The Way for Advanced Applications of Smart Materials. Coord. Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Jain, P.; Sharma, M.; Dureja, P.; Sarma, P.M.; Lal, B. Bioelectrochemical approaches for removal of sulfate, hydrocarbon, and salinity from produced water. Chemosphere 2017, 166, 96–108. [Google Scholar] [CrossRef]
- Ghafoori, S.; Omar, M.; Koutahzadeh, N.; Zendehboudi, S.; Malhas, R.N.; Mohamed, M.; Al-Zubaidi, S.; Redha, K.; Baraki, F.; Mehrvar, M. New advancements, challenges, and future needs on treatment of oilfield produced water: A state-of-the-art review. Sep. Purif. Technol. 2022, 289, 120652. [Google Scholar] [CrossRef]
- Michael-Kordatou, I.; Michael, C.; Duan, X.; He, X.; Dionysiou, D.D.; Mills, M.A.; Fatta-Kassinos, D. Dissolved Effluent Organic Matter: Characteristics and Potential Implications in Wastewater Treatment and Reuse Applications. Water Res. 2015, 77, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Subramani, A.; Jacangelo, J.G. Emerging desalination technologies for water treatment: A critical review. Water Res. 2015, 75, 164–187. [Google Scholar] [CrossRef]
- Khor, C.M.; Wang, J.; Li, M.; Oettel, B.A.; Kaner, R.B.; Jassby, D.; Hoek, E.M.V. Performance, energy and cost of produced water treatment by chemical and electrochemical coagulation. Water 2020, 12, 3426. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Produced water treatment: Application of Air Gap Membrane Distillation. Desalination 2013, 309, 46–51. [Google Scholar] [CrossRef]
- Xie, Z.; Diao, S.; Xu, R.; Wei, G.; Wen, J.; Hu, G.; Tang, T.; Jiang, L.; Li, M.; Huang, H. Construction of carboxylated-GO and MOFs composites for efficient removal of heavy metal ions. Appl. Surf. Sci. 2023, 636, 157827. [Google Scholar] [CrossRef]
- Xu, R.; Wei, G.; Xie, G.; Diao, S.; Wen, J.; Tang, T.; Jiang, L.; Li, M.; Hu, G. V2C MXene–modified g-C3N4 for enhanced visible-light photocatalytic activity. J. Alloys Compd. 2023, 970, 172656. [Google Scholar] [CrossRef]
- Darling, S.B. Perspective: Interfacial materials at the interface of energy and water. J. Appl. Phys. 2018, 124, 030901. [Google Scholar] [CrossRef]
- da Motta, A.R.P.; Borges, C.P.; Kiperstok, A.; Esquerre, K.P.; Araujo, P.M.; da Paz Nogueira Branco, L. Treatment of petroleum produced water for oil removal by membrane separation processes: Review. Sanit. Environ. Eng. 2013, 18, 15–26. [Google Scholar] [CrossRef]
- Weschenfelder, S.E.; Mello, A.C.C.; Borges, C.P.; Campos, J.C. Oilfield produced water treatment by ceramic membranes: Preliminary process cost estimation. Desalination 2015, 360, 81–86. [Google Scholar] [CrossRef]
- Chang, H.; Li, T.; Liu, B.; Vidic, R.D.; Elimelech, M.; Crittenden, J.C. Potential and implemented membrane-based technologies for the treatment and reuse of flowback and produced water from shale gas and oil plays: A review. Desalination 2019, 455, 34–57. [Google Scholar] [CrossRef]
- Garland, E. Environmental regulatory framework in Europe: An update. In Proceedings of the SPE/EPA/DOE Exploration and Production Environmental Conference, Galveston, TX, USA, 7–9 March 2005. [Google Scholar]
- Shaffer, D.L.; Arias Chavez, L.H.; Ben-Sasson, M.; Romero-Vargas Castrillón, S.; Yip, N.Y.; Elimelech, M. Desalination and reuse of high-salinity shale gas produced water: Drivers, technologies, and future directions. Environ. Sci. Technol. 2013, 47, 9569–9583. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Chen, L.; Xu, P.; Wang, H. Treatment of produced water with photocatalysis: Recent advances, affecting factors and future research prospects. Catalysts 2020, 10, 924. [Google Scholar] [CrossRef]
- Xiao, F. Characterization, and treatment of Bakken oilfield produced water as a potential source of value-added elements. Sci. Total Environ. 2021, 770, 145283. [Google Scholar] [CrossRef]














| Method | Target of Removal | Pros | Cons | Results | References |
|---|---|---|---|---|---|
| Hydrocyclone | 5–15 µm suspended Solid, Dispersed, and free oil in PW | (1) Does not require moving different parts (2) Pretreatment process is not required | (1) Cleaning and proper maintenance are required (2) Solids may clog the inlet system | (1) More than 90% separation rate (2) 60 mg/L oil concentration in the contaminated water can be removed (3) Hydraulic resistance time is short | [8,134,135] |
| Thermal Separation process | different stages according to the boiling point of these materials. | Readily available and cost-effective for the Middle Eastern region | High instrumentation is required, and it is hard to control the process | More than 90 percent of organic substances can be removed | [10,141,171] |
| Adsorption | Most of the pollutants in PW | Treatment efficiency is good; water recovery is approximately 100% | Phase-transferred contaminants require secondary treatment | Lowering initial oil concentration, volumetric flow rate, particle size, and bed height increases oil removal rate | [143,144,145,146,147,148] |
| Gravity Separation | Large SS; Free and dispersed oil. | (1) Low-cost, easy operation (2) High processing power (3) Stable treatment efficacy (4) Chemical-free | (1) Big footprint and expensive startup (2) Retention duration increases with smaller oil droplets | (1) >99% dehydration (2) Treatment capacity increased >350% | [148,152,153,154] |
| Flotation | Dispersed and emulsified oil (0.25–25 μm) | (1) Mature change (2) Good effectiveness of treatment | It cannot treat greasy wastewater with various oils | (1) <50 mg/L oil concentration (2) Bicyclone and dissolved air flotation devices produce effluent with oil droplet sizes of 3.97 μm and 7.21 μm, respectively | [154,155,156,157,158,159,160,161,163,164,172,173,174] |
| Coalescence separation | Emulsified oil | (1) Compact build (2) Very accurate separation (3) Long-lasting coalescing materials | Solid particles and sludge might clog coalescence layer | After 180 s and 30 psi pressure reduction, oil pollutant concentration dropped from 1200 to 25 mg/dl | [165,167] |
| Filtration separation | Large SS; Oil | (1) Low-cost, easy operation (2) Effective therapy (3) Backwash-friendly (4) Salinity is ineffective | Filter medium can be blocked, and backwashing is needed | (1) Over 85% of oil and suspended particles are removed (2) Greater than 98.8% filter material generation | [162,169] |
| Methods | Target of Removal | Pros | Cons | Results | References |
|---|---|---|---|---|---|
| Thermal | Salts | Long-lasting, mature, sturdy, easy to use, suited for high-TDS samples. | Reduced recovery rate, corrosion and scale inhibitors needed, energy usage. | Simple in use | [211,212] |
| Biological | BTEX, TDS, SS, organics | Low-maintenance, cost-effective, high-water recovery | High retention time, requires sludge disposal | Cost-effective | [137,213] |
| Membrane Distillation | TDS, dissolved organics, salts, hydrocarbons | Compact, automated, high pH tolerance, removes dissolved impurities and monovalent salts, energy-efficient, chemical-free, greater capital cost than microfiltration and nanofiltration. | Membrane fouling, additional waste formation, high pressure, and demand for more energy than nanofiltration | Excellent in automation | [178,180,211,214,215,216,217,218,219] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nath, F.; Chowdhury, M.O.S.; Rhaman, M.M. Navigating Produced Water Sustainability in the Oil and Gas Sector: A Critical Review of Reuse Challenges, Treatment Technologies, and Prospects Ahead. Water 2023, 15, 4088. https://doi.org/10.3390/w15234088
Nath F, Chowdhury MOS, Rhaman MM. Navigating Produced Water Sustainability in the Oil and Gas Sector: A Critical Review of Reuse Challenges, Treatment Technologies, and Prospects Ahead. Water. 2023; 15(23):4088. https://doi.org/10.3390/w15234088
Chicago/Turabian StyleNath, Fatick, Mohammed Omar Sahed Chowdhury, and Md. Masudur Rhaman. 2023. "Navigating Produced Water Sustainability in the Oil and Gas Sector: A Critical Review of Reuse Challenges, Treatment Technologies, and Prospects Ahead" Water 15, no. 23: 4088. https://doi.org/10.3390/w15234088