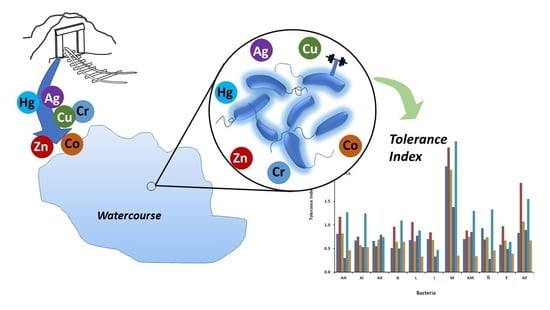
Evidence of Resistance of Heavy Metals from Bacteria Isolated from Natural Waters of a Mining Area in Mexico
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Gathering
2.2. Identification of Bacteria Tolerance to Heavy Metals
2.3. Assessment of Metal Toxicity
2.4. Determination of Kinetic Parameters and Tolerance Index
3. Results and Discussion
3.1. Water Characteristics
3.2. Isolated Bacteria
3.3. Heavy Metal Resistance Evaluation
- (A)
- Susceptible, if the bacteria growth was inhibited by the tested concentration, which is characterized by an inhibitory zone higher than 18 mm,
- (B)
- Resistant, if the bacteria growth persisted in the presence of heavy metal ions; that is: if they showed an inhibitory zone lower than 13 mm,
- (C)
- Intermediate, if the bacteria showed an inhibitory zone between 13–18 mm, which indicates the bacteria metal tolerance [39],
- (D)
- Susceptible Dose-Dependent (SDD), this term is related to those bacteria without an inhibitory zone; that means a higher concentration of a heavy metal solution is necessary to determine the Minimum Inhibitory Concentration (MIC), as shown in Table 3. The MIC is defined as the lowest metal concentration, which completely averts bacterial growth (the presence of an inhibitory zone).
3.4. Metal Tolerance Index
3.5. Kinetic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahluwalia, S.S.; Goyal, D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresour. Technol. 2007, 98, 2243–2257. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mazumder, M.A.J.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents-A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Pérez Castresana, G.; Castañeda Roldán, E.; García Suastegui, W.A.; Morán Perales, J.L.; Cruz Montalvo, A.; Handal Silva, A. Evaluation of health risks due to heavy metals in a rural population exposed to Atoyac River pollution in Puebla, Mexico. Water 2019, 11, 277. [Google Scholar] [CrossRef] [Green Version]
- Razo, I.; Carrizales, L.; Castro, J.; Díaz-Barriga, F.; Monroy, M. Arsecin and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water. Air. Soil Pollut. 2004, 152, 129–152. [Google Scholar] [CrossRef]
- García-Hernández, J.; Cadena-Cárdenas, L.; Betancourt-Lozano, M.; García-De-La-Parra, L.M.; García-Rico, L.; Márquez-Farías, F. Total mercury content found in edible tissues of top predator fish from the Gulf of California, Mexico. Toxicol. Environ. Chem. 2007, 89, 507–522. [Google Scholar] [CrossRef]
- Covarrubias, S.A.; Peña Cabriales, J.J. Contaminación ambiental por metales pesados en México: Problemática y estrategias de fitorremediación. Rev. Int. Contam. Ambient. 2017, 33, 7–21. [Google Scholar] [CrossRef]
- Gómez-Alvarez, A.; Meza-Figueroa, D.; Villalba-Atondo, A.I.; Valenzuela-García, J.L.; Ramírez-Hernández, J.; Almendariz-Tapia, J. Estimation of potential pollution from mine tailings in the San Pedro River (1993–2005), Mexico-US border. Environ. Geol. 2009, 57, 1469–1479. [Google Scholar] [CrossRef]
- Levresse, G.; Lopez, G.; Tritlla, J.; López, E.C.; Chavez, A.C.; Salvador, E.M.; Soler, A.; Corbella, M.; Sandoval, L.G.H.; Corona-Esquivel, R. Phytoavailability of antimony and heavy metals in arid regions: The case of the Wadley Sb district (San Luis, Potosí, Mexico). Sci. Total Environ. 2012, 427–428, 115–125. [Google Scholar] [CrossRef]
- Lizárraga-Mendiola, L.; González-Sandoval, M.R.; Durán-Domínguez, M.C.; Márquez-Herrera, C. Geochemical behavior of heavy metals in a Zn-Pb-Cu mining area in the State of Mexico (central Mexico). Environ. Monit. Assess. 2009, 155, 355–372. [Google Scholar] [CrossRef]
- Vdovin, V.; Pareja, G.; Lind, P. Peñasquito Polymetallic Operation; Zacatecas State Mexico; NI 43-101 Technical Report; Goldcorp Inc.: Vancouver, BC, Canada, 2010. [Google Scholar]
- Joseph, L.; Jun, B.M.; Flora, J.R.V.; Park, C.M.; Yoon, Y. Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere 2019, 229, 142–159. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Venkata Mohan, S.; Lens, P.N.L. Metals removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2015, 195, 102–114. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Sharma, S.K. Heavy Metals in Water; Royal Society of Chemistry: Cambridge, UK, 2014; ISBN 9781782620174. [Google Scholar]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Banerjee, G.; Pandey, S.; Ray, A.K.; Kumar, R. Bioremediation of heavy metals by a novel bacterial strain Enterobacter cloacae and its antioxidant enzyme activity, flocculant production, and protein expression in presence of lead, cadmium, and nickel. Water. Air. Soil Pollut. 2015, 226, 1–9. [Google Scholar] [CrossRef]
- De Niederhäusern, S.; Bondi, M.; Anacarso, I.; Iseppi, R.; Sabia, C.; Bitonte, F.; Messi, P. Antibiotics and heavy metals resistance and other biological characters in enterococci isolated from surface water of Monte Cotugno Lake (Italy). J. Environ. Sci. Health—Part A Toxic/Hazardous Subst. Environ. Eng. 2013, 48, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Paul, A.K. Hexavalent chromium reduction by aerobic heterotrophic bacteria indigenous to chromite mine overburden. Braz. J. Microbiol. 2013, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Oyetibo, G.O.; Miyauchi, K.; Huang, Y.; Chien, M.F.; Ilori, M.O.; Amund, O.O.; Endo, G. Biotechnological remedies for the estuarine environment polluted with heavy metals and persistent organic pollutants. Int. Biodeterior. Biodegrad. 2017, 119, 614–625. [Google Scholar] [CrossRef]
- Qin, H.; Hu, T.; Zhai, Y.; Lu, N.; Aliyeva, J. The improved methods of heavy metals removal by biosorbents: A review. Environ. Pollut. 2020, 258, 113777. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef]
- Won, S.W.; Kotte, P.; Wei, W.; Lim, A.; Yun, Y.S. Biosorbents for recovery of precious metals. Bioresour. Technol. 2014, 160, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Rathna, R.; Nakkeeran, E. Biological Treatment for the Recovery of Minerals from Low-Grade Ores; Elsevier B.V.: Amsterdam, The Netherlands, 2020; ISBN 9780444643216. [Google Scholar]
- Işıldar, A.; van Hullebusch, E.D.; Lenz, M.; Du Laing, G.; Marra, A.; Cesaro, A.; Panda, S.; Akcil, A.; Kucuker, M.A.; Kuchta, K. Biotechnological strategies for the recovery of valuable and critical raw materials from waste electrical and electronic equipment (WEEE)—A review. J. Hazard. Mater. 2019, 362, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Christopher, M.; Paul, O.; Hamadi, B. Association of metal tolerance with multidrug resistance among environmental bacteria from wetlands of Lake Victoria basin. Agric. Biol. J. N. Am. 2014, 5, 24–32. [Google Scholar] [CrossRef]
- Barkay, T. Adaptation of Aquatic Microbial Communities to Hg 2+ Stress. Appl. Environ. Microbiol. 1987, 53, 2725–2732. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Tripathi, S.; Chandra, R. Metagenomic analysis for profiling of microbial communities and tolerance in metal-polluted pulp and paper industry wastewater. Bioresour. Technol. 2021, 324, 124681. [Google Scholar] [CrossRef]
- Naguib, M.M.; Khairalla, A.S.; El-Gendy, A.O.; Elkhatib, W.F. Isolation and characterization of mercury-resistant bacteria from wastewater sources in egypt. Can. J. Microbiol. 2019, 65, 308–321. [Google Scholar] [CrossRef]
- Cai, X.; Zheng, X.; Zhang, D.; Iqbal, W.; Liu, C.; Yang, B.; Zhao, X.; Lu, X.; Mao, Y. Microbial characterization of heavy metal resistant bacterial strains isolated from an electroplating wastewater treatment plant. Ecotoxicol. Environ. Saf. 2019, 181, 472–480. [Google Scholar] [CrossRef]
- Lima e Silva, A.A.; Carvalho, M.A.; de Souza, S.A.; Dias, P.M.; Silva Filho, R.G.; Saramago, C.S.; Bento, C.A.; Hofer, E. Heavy metal tolerance (Cr, Ag and Hg) in bacteria isolated from sewage. Braz. J. Microbiol. 2012, 43, 1620–1631. [Google Scholar] [CrossRef] [Green Version]
- Nokman, W.; Benluvankar, V.; Maria Packiam, S.; Vincent, S. Screening and molecular identification of heavy metal resistant Pseudomonas putida S4 in tannery effluent wastewater. Biocatal. Agric. Biotechnol. 2019, 18, 101052. [Google Scholar] [CrossRef]
- Secretaría de Comercio y Fomento Industrial. Residual Waters—Sampling; NMX-AA-003-1980; Diario Oficial de la Federación: Mexico City, Mexico, 1980; p. 9.
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Anionic Surfactants as MBAS. In Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1999; pp. 1027–1033. [Google Scholar]
- Secretaría de Economía. Water Analisis—Determination of Metals by Atomic Absorption in Natural, Drinking, Wastewaters and Wastewaters Treated—Test Method; NMX-AA-051-SCFI-2001; Diario Oficial de la Federación: Mexico City, Mexico, 2001.
- Secretaría de Salud. Salud Ambiental, Agua para Uso y Consumo Humano-Limites Permisibles de Calidad y Tratamientos a que Debe Someterse el Agua para su Potabilización; NOM-127-SSA1-1994; Diario Oficial de la Federación: Mexico City, Mexico, 1994.
- Hassen, A.; Saidi, N.; Cherif, M.; Boudabous, A. Effects of heavy metals on Pseudomonas aeruginosa and Bacillus thuringiensis. Bioresour. Technol. 1998, 65, 73–82. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single dosk method. Am. J. Clin. Pathol. 1966, 36, 493–496. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Patel, J.B.; Alder, J.; Brandford, P.B.; Dudley, M.N.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Powell, M.; Swenson, J.M.; et al. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2013; ISBN 1562388975. [Google Scholar]
- Krumperman, P.H. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of faecal contamination of foods. Appl. Environ. Microbiol. 1983, 46, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Matyar, F.; Kaya, A.; Dinçer, S. Antibacterial agents and heavy metal resistance in Gram-negative bacteria isolated from seawater, shrimp and sediment in Iskenderun Bay, Turkey. Sci. Total Environ. 2008, 407, 279–285. [Google Scholar] [CrossRef]
- Kimiran-Erdem, A.; Arslan-Aydoğdu, E.Ö.; Gürün, S.; Altun, Ö. Determination of multiple antibiotic and heavy metal resistance of the bacteria isolated from the Küçükçekmece Lagoon, Turkey. Polish J. Environ. Stud. 2015, 24, 1077–1084. [Google Scholar] [CrossRef]
- Muñoz, A.J.; Ruiz, E.; Abriouel, H.; Gálvez, A.; Ezzouhri, L.; Lairini, K.; Espínola, F. Heavy metal tolerance of microorganisms isolated from wastewaters: Identification and evaluation of its potential for biosorption. Chem. Eng. J. 2012, 210, 325–332. [Google Scholar] [CrossRef]
- Secretaría de Ecología. Que Establece los Límites Máximos Permisibles de Contaminantes en las Desacrgas de Aguas Residuales en Aguas y Bienes Nacionales; NOM-001-ECOL-1996; Diario Oficial de la Federación: Mexico City, Mexico, 1996; p. 13.
- Roane, T.M.; Kellogg, S.T. Characterization of bacterial communities in heavy metal contaminated soils. Can. J. Microbiol. 1996, 42, 593–603. [Google Scholar] [CrossRef]
- Nithya, C.; Gnanalakshmi, B.; Pandian, S.K. Assessment and characterization of heavy metal resistance in Palk Bay sediment bacteria. Mar. Environ. Res. 2011, 71, 283–294. [Google Scholar] [CrossRef]
- Karadede, H.; Ünlü, E. Concentrations of some heavy metals in water, sediment and fish species from the Ataturk Dam Lake (Euphrates), Turkey. Chemosphere 2000, 41, 1371–1376. [Google Scholar] [CrossRef]
- Gibson, B.; Wilson, D.J.; Feil, E.; Eyre-Walker, A. The distribution of bacterial doubling times in the wild. Proc. R. Soc. B Biol. Sci. 2018, 285. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Loukidou, M.X.; Matis, K.A. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem. 2004, 39, 909–916. [Google Scholar] [CrossRef]
- Singleton, F.L.; Guthrie, R.K. Aquatic bacterial populations and heavy metals-I. Composition of aquatic bacteria in the presence of copper and mercury salts. Water Res. 1977, 11, 639–642. [Google Scholar] [CrossRef]
- Sterritt, R.M.; Lester, J.N. Interactions of heavy metals with minerals. Sci. Total Environ. 1980, 14, 5–17. [Google Scholar] [CrossRef]
- Ezzouhri, L.; Castro, E.; Moya, M.; Espinola, F.; Lairini, K. Heavy metal tolerance of filamentous fungi isolated from polluted sites in Tangier, Morocco. Afr. J. Microbiol. Res. 2009, 3, 35–48. [Google Scholar]
- Das, N. Recovery of precious metals through biosorption—A review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Priyadarshanee, M.; Das, S. Biosorption and removal of toxic heavy metals by metal tolerating bacteria for bioremediation of metal contamination: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 104686. [Google Scholar] [CrossRef]
- Florence, T.M. The Speciation of trace eElements in waters. Talanta 1982, 29, 345–364. [Google Scholar] [CrossRef]
- Duxbury, T.; Bicknell, B. Metal-tolerant bacterial populations from natural and metal-polluted soils. Soil Biol. Biochem. 1983, 15, 243–250. [Google Scholar] [CrossRef]
- Rajbanshi, A. Study on Heavy Metal Resistant Bacteria in Guheswori Sewage Treatment Plant. Our Nat. 2008, 6, 52–57. [Google Scholar] [CrossRef]
- Malik, A. Metal bioremediation through growing cells. Environ. Int. 2004, 30, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.I.; Malik, A. Biosorption of nickel and cadmium by metal resistant bacterial isolates from agricultural soil irrigated with industrial wastewater. Bioresour. Technol. 2007, 98, 3149–3153. [Google Scholar] [CrossRef] [PubMed]
- Beyenal, N.Y.; Özbelge, T.A.; Özbelge, H.Ö. Combined effects of Cu2+ and Zn2+ on activated sludge process. Water Res. 1997, 31, 699–704. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and bioaccumulation-the prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Mclean, J.; Beveridge, T.J. Chromate Reduction by a Pseudomonad Isolated from a Site Contaminated with Chromated Copper Arsenate Chromate Reduction by a Pseudomonad Isolated from a Site Contaminated with Chromated Copper Arsenate. Appl. Environ. Microbiol. 2001, 67, 1076–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Sample Origin | pH | Temp. (°C) | Total CFU/mL | Coliforms CFU/mL | Hardness (ppm CaCO3) | Alkalinity (ppm) |
|---|---|---|---|---|---|---|
| Spring | 7.4 | 10.0 | 1.89 × 104 | 2.00 × 102 | 150 | 300 |
| Intake | 7.7 | 9.5 | 3.00 × 103 | 2.80 × 103 | 150 | 300 |
| Stream | 8.1 | 10.0 | C | 2.00 × 103 | 150 | 180 |
| Tank | 7.6 | 10.5 | 1.04 × 105 | 1.00 × 102 | 150 | 300 |
| Overflow | 8.1 | 10.5 | C | 1.05 × 104 | 1000 | 300 |
| Overflow | 7.2 | 10.6 | C | 0 | 150 | 300 |
| Dam | 7.3 | 11.3 | 1.42 × 105 | 0 | 300 | 300 |
| Dam | 7.9 | 11.6 | C | 0 | 150 | 300 |
| Dam | 6.9 | 13.0 | C | 0 | 150 | 300 |
| Dam | 7 | 11.3 | 7.20 × 103 | 0 | 150 | 300 |
| Dam | 7 | 12.3 | 1.23 × 104 | 0 | 150 | 720 |
| Dam | 8 | 7.6 | 2.60 × 103 | 0 | 300 | 300 |
| Dam | 8 | 13.3 | 1.65 × 104 | 0 | 150 | 180 |
| Dam | 7 | 12.3 | C | 0 | 1000 | 300 |
| Bacteria Code | Growth % | Primary ID | |||||
|---|---|---|---|---|---|---|---|
| Cr | Zn | Cu | Ag | Hg | Co | ||
| AE | 100 | 120 | 100 | 140 | 120 | 20 | Staphylococcus spp |
| AI | 200 | 150 | 125 | 175 | 125 | 0 | Enterobacteriaceae |
| AK | 175 | 125 | 175 | 175 | 150 | 0 | Pseudomonas spp |
| AN | 150 | 250 | 175 | 225 | 100 | 50 | No identified |
| AP | 143 | 143 | 143 | 143 | 100 | 14.3 | Enterobacteriaceae |
| AQ | 100 | 160 | 140 | 200 | 100 | 20 | Gram (-) bacterium |
| G | 167 | 117 | 100 | 133 | 66.7 | 66.7 | Staphylococcus spp |
| L | 73 | 100 | 136 | 136 | 109 | 0 | Pseudomonas spp |
| AG | 200 | 167 | 67 | 233 | 133 | 0 | No identified |
| AH | 100 | 60 | 140 | 120 | 160 | 0 | Enterobacteriaceae |
| AR | 86 | 100 | 100 | 143 | 100 | 71.4 | Enterobacteriaceae |
| J | 100 | 85.7 | 143 | 114 | 42.9 | 0 | Enterobacteriaceae |
| Q | 71 | 129 | 114 | 129 | 71.4 | 0 | Enterobacteriaceae |
| Z | 56 | 122 | 100 | 111 | 88.9 | 77.8 | Enterobacteriaceae |
| I | 50 | 125 | 75 | 100 | 37.5 | 25 | Enterobacteriaceae |
| K | 100 | 60 | 140 | 60 | 0 | 0 | Enterobacteriaceae |
| M | 117 | 83.3 | 167 | 83 | 50 | 0 | Pseudomonas spp |
| N | 100 | 71.4 | 86 | 100 | 14.3 | 42.9 | Pseudomonas spp |
| Y | 56 | 55.6 | 44 | 111 | 100 | 55.6 | Enterobacteriaceae |
| AM | 86 | 71.9 | 71 | 129 | 114 | 28.6 | Pseudomonas spp |
| AÑ | 0 | 120 | 100 | 40 | 0 | 0 | No identified |
| A | 56 | 77.8 | 67 | 111 | 55.6 | 55.6 | Pseudomonas spp |
| Ñ | 129 | 85.7 | 86 | 86 | 28.6 | 0 | Pseudomonas spp |
| V | 71 | 71.4 | 100 | 57 | 7.14 | 0 | No identified |
| X | 78 | 66.7 | 67 | 122 | 55.6 | 55.6 | Pseudomonas spp |
| AC | 85 | 115 | 92 | 85 | 53.8 | 0 | No identified |
| AD | 83 | 117 | 83 | 83 | 83.3 | 0 | No identified |
| AO | 0 | 42.9 | 100 | 43 | 14.3 | 71.4 | No identified |
| F | 71 | 85.7 | 43 | 71 | 57.1 | 57.1 | Staphylococcus spp |
| H | 67 | 33.3 | 67 | 100 | 55.6 | 55.6 | Pseudomonas spp |
| AF | 83 | 66.7 | 83 | 83 | 83.3 | 50 | Enterobacteriaceae |
| Bacteria | MIC (mg L−1) | MMR | |||||
|---|---|---|---|---|---|---|---|
| Cr | Cu | Zn | Ag | Hg | Co | ||
| AE | SDD, I | SDD, I | 260, R | SDD, I | 65, R | 650, I | 0.33 |
| AI | 650, I | SDD, I | 2500, R | SDD, I | 65, R | 65, R | 0.50 |
| AK | SDD, I | SDD, I | 2500, R | SDD, I | 65, R | 650, R | 0.50 |
| AN | SDD, I | SDD, I | SDD, I | SDD, I | 65, R | 1250, I | 0.16 |
| AP | SDD, I | SDD, I | SDD, I | SDD, I | 65, R | SDD, I | 0.16 |
| G | 650, S | SDD, I | 2500, R | SDD, I | 65, R | 650, R | 0.50 |
| L | SDD, I | SDD, I | 2500, R | SDD, I | 65, R | 105, R | 0.50 |
| AG | 650, S | SDD, I | 650, I | SDD, I | 65, R | 650, I | 0.16 |
| AH | SDD, I | SDD, I | 2500, R | 2500, R | 85, R | 105, R | 0.66 |
| AR | 260, S | SDD, I | 2500, R | SDD, I | 65, R | SDD, I | 0.33 |
| J | 650, R | SDD, I | SDD, I | SDD, I | 85, R | SDD, I | 0.33 |
| K | SDD, I | SDD, I | SDD, I | SDD, I | 85, R | SDD, I | 0.16 |
| Z | 650, S | SDD, I | SDD, I | SDD, I | 85, R | SDD, I | 0.16 |
| I | SDD, I | SDD, I | 2500, R | SDD, I | 85, R | 1250, R | 0.50 |
| M | 650, I | SDD, I | 2500, R | SDD, I | 65, R | 65, R | 0.50 |
| N | SDD, I | SDD, I | 2500, R | SDD, I | 85, R | 1250, R | 0.50 |
| Y | 650, I | SDD, I | 2500, R | SDD, I | 65, R | SDD, I | 0.33 |
| AM | 650, I | SDD, I | 2500, R | SDD, I | 85, R | 650, R | 0.50 |
| AÑ | SDD, I | SDD, I | 2500, R | SDD, I | 65, R | 105, I | 0.33 |
| A | SDD, I | SDD, I | 650, R | SDD, I | 85, R | 650, I | 0.33 |
| Ñ | 650, I | SDD, I | 2500, R | SDD, I | 65, R | 650, R | 0.50 |
| X | 1250, R | SDD, I | 2500, R | SDD, I | 85, R | SDD, I | 0.50 |
| AC | SDD, I | SDD, I | 2500, R | SDD, I | 65, R | 650, I | 0.33 |
| AD | 1250, R | SDD, I | 2500, R | SDD, I | 65, R | 650, I | 0.50 |
| AO | 650, I | SDD, I | 650, S | SDD, I | 85, I | 650, S | NA |
| F | SDD, I | SDD, I | 2500, R | SDD, I | 85, R | 650, R | 0.50 |
| H | SDD, I | SDD, I | SDD, I | SDD, I | 85, R | 105, I | 0.16 |
| AF | SDD, I | SDD, I | 2500, R | SDD, I | 85, R | 650, R | 0.50 |
| Bacteria | Metal-Free | Cr | Zn | Cu | Ag | Hg | Co | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| µ | td | µ | td | µ | td | µ | td | µ | td | µ | td | µ | td | |
| AH | 0.03 | 23.5 | 0.03 | 23.3 | 0.03 | 23.3 | 0.029 | 23.7 | 0.03 | 23.4 | 0.034 | 20.6 | 0.029 | 23.9 |
| AI | 0.027 | 26 | 0.031 | 22.5 | 0.017 | 40.8 | 0.031 | 22.3 | 0.035 | 19.8 | 0.029 | 24.2 | 0.028 | 24.3 |
| AK | 0.032 | 21.5 | 0.033 | 20.9 | 0.028 | 24.4 | 0.024 | 28.4 | 0.033 | 21.1 | 0.029 | 24.2 | 0.024 | 28.3 |
| G | 0.03 | 22.9 | 0.028 | 24.4 | 0.028 | 25 | 0.028 | 25.2 | 0.026 | 26.5 | 0.016 | 43.3 | 0.081 | 8.5 |
| L | 0.029 | 24.1 | 0.028 | 24.7 | 0.027 | 25.3 | 0.026 | 26.3 | 0.033 | 21.1 | 0.027 | 25.4 | 0.027 | 25.6 |
| I | 0.032 | 22 | 0.027 | 25.3 | 0.029 | 23.6 | 0.029 | 23.9 | 0.028 | 25 | 0.027 | 25.3 | 0 | 0 |
| M | 0.027 | 25.4 | 0.027 | 25.7 | 0.029 | 24.2 | 0.027 | 25.8 | 0.028 | 25 | 0.028 | 24.5 | 0.03 | 23.4 |
| AM | 0.028 | 24.6 | 0.028 | 24.7 | 0.028 | 24.9 | 0.028 | 24.9 | 0.026 | 26.2 | 0.03 | 23.3 | 0.027 | 25.3 |
| Ñ | 0.026 | 26.9 | 0.034 | 20.5 | 0.034 | 20.5 | 0.031 | 22.3 | 0.028 | 24.6 | 0.033 | 21.2 | 0.026 | 26.3 |
| F | 0.026 | 26.6 | 0.027 | 25.4 | 0.028 | 25 | 0.03 | 23.2 | 0.026 | 26.4 | 0.026 | 26.2 | 0.027 | 25.3 |
| AF | 0.027 | 25.5 | 0.027 | 25.5 | 0.028 | 25.1 | 0.027 | 25.3 | 0.031 | 22.1 | 0.027 | 25.4 | 0.026 | 27.0 |
| Bacteria | Inhibited Growth Curve (c) | Without Effect Growth Curve (b) | Considerable Inhibited Growth (d) | Identification |
|---|---|---|---|---|
| AI | -- | Cr, Cu, Ag, Hg, Co | Zn | Pseudomonas koreensis |
| L | -- | Ag | Cr, Zn, Cu, Hg, Co | Pseudomonas azotoformans |
| AM | Cr, Zn, Cu | Hg | Ag, Co | Pseudomonas fluorences |
| Ñ | Co | Cr, Zn, Cu, Ag, Hg | -- | Pseudomonas koreensis |
| AH | Zn, Cr, Ag | Hg | Cu, Co | Not determined |
| AK | -- | Cr, Ag | Zn, Cu, Hg, Co | Pseudomonas azotoformans |
| G | -- | Co | Cr, Zn, Cu, Ag, Hg | Not determined |
| M | Zn, Ag, Co | Cr, Cu | Hg | Not determined |
| F | Ag, Hg | Cr, Zn, Cu, Co | -- | Not determined |
| I | -- | -- | Cr, Zn, Cu, Ag, Hg, Co | Not determined |
| AF | Cr, Cu, Hg | Zn, Ag | Co | Not determined |
| Bacteria | Genus | MMR | 1/tdm | Tolerance Index | PI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Zn | Cu | Ag | Hg | Co | |||||
| M | Pseudomonas spp | 0.5 | 0.040 | 2.24 | 2.64 | 2.17 | 1.38 | 2.77 | 0.35 | 0.23 |
| AH | Enterobacteriaceae | 0.66 | 0.043 | 0.81 | 1.17 | 0.82 | 0.3 | 1.27 | 0.46 | 0.14 |
| AF | Enterobacteriaceae | 0.5 | 0.040 | 0.83 | 1.89 | 1.07 | 0.89 | 1.55 | 0.67 | 0.14 |
| Ñ | Pseudomonas spp | 0.5 | 0.044 | 0.93 | 0.69 | 0.74 | 0.28 | 1.33 | 0.46 | 0.10 |
| AM | Pseudomonas spp | 0.5 | 0.040 | 0.7 | 0.88 | 0.75 | 0.85 | 1.3 | 0.34 | 0.10 |
| L | Pseudomona spp | 0.5 | 0.040 | 0.68 | 1.06 | 0.65 | 0.77 | 0.88 | 0.33 | 0.09 |
| G | Staphylococcus spp | 0.5 | 0.039 | 0.51 | 0.96 | 0.64 | 0.5 | 1.09 | 0.64 | 0.09 |
| AI | Enterobacteriaceae | 0.5 | 0.039 | 0.67 | 0.75 | 0.57 | 0.53 | 1.24 | 0.53 | 0.08 |
| F | Staphylococcus spp | 0.5 | 0.040 | 0.58 | 0.97 | 0.67 | 0.49 | 0.64 | 0.39 | 0.07 |
| I | Enterobacteriaceae | 0.5 | 0.049 | 0.7 | 0.84 | 0.68 | 0.33 | 0.47 | 0 | 0.07 |
| K | Enterobacteriaceae | 0.5 | 0.041 | 0.66 | 0.55 | 0.69 | 0.79 | 0.74 | 0.01 | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escamilla-Rodríguez, A.; Carlos-Hernández, S.; Díaz-Jiménez, L. Evidence of Resistance of Heavy Metals from Bacteria Isolated from Natural Waters of a Mining Area in Mexico. Water 2021, 13, 2766. https://doi.org/10.3390/w13192766
Escamilla-Rodríguez A, Carlos-Hernández S, Díaz-Jiménez L. Evidence of Resistance of Heavy Metals from Bacteria Isolated from Natural Waters of a Mining Area in Mexico. Water. 2021; 13(19):2766. https://doi.org/10.3390/w13192766
Chicago/Turabian StyleEscamilla-Rodríguez, Alondra, Salvador Carlos-Hernández, and Lourdes Díaz-Jiménez. 2021. "Evidence of Resistance of Heavy Metals from Bacteria Isolated from Natural Waters of a Mining Area in Mexico" Water 13, no. 19: 2766. https://doi.org/10.3390/w13192766