Molecular Dications in Planetary Atmospheric Escape
Abstract
:1. Introduction
2. Experiments
3. Results and Discussion
3.1. The Double Photoionization of CO2 and the Lack of O+ Concentration in Mars Atmosphere
3.2. The Coulomb Explosion of Nitrous Oxide Dication
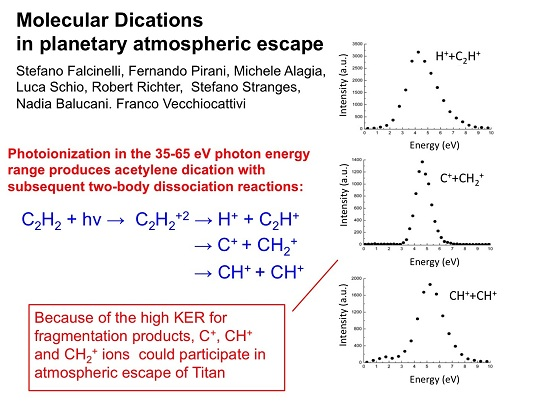
3.3. The Coulomb Explosion of Acetylene Dication
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| KER | Kinetic Energy Released |
| VUV | Vacuum Ultraviolet |
| UV | Ultraviolet |
| ARPES | Angle-Resolved Photoemission Spectroscopy |
References
- Balucani, N.; Bartocci, A.; Brunetti, B.G.; Candori, P.; Falcinelli, S.; Pirani, F.; Palazzetti, F.; Vecchiocattivi, F. Collisional autoionization dynamics of Ne*(3P2,0)-H2O. Chem. Phys. Lett. 2012, 546, 34–39. [Google Scholar] [CrossRef]
- Brunetti, B.G.; Candori, P.; Cappelletti, D.; Falcinelli, S.; Pirani, F.; Stranges, D.; Vecchiocattivi, F. Penning Ionization Electron Spectroscopy of water molecules by metastable neon atoms. Chem. Phys. Lett. 2012. [Google Scholar] [CrossRef]
- Falcinelli, S.; Bartocci, A.; Cavalli, S.; Pirani, F.; Vecchiocattivi, F. The stereo-dynamics of collisional autoionization of ammonia by helium and neon metastable excited atoms through molecular beam experiments. J. Chem. Phys. 2015. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, S.; Bartocci, A.; Cavalli, S.; Pirani, F.; Vecchiocattivi, F. Stereo-dynamics in collisional autoionization of water, ammonia, and hydrogen sulfide with metastable rare gas atoms: Competition between intermolecular halogen and hydrogen bonds. Chem. Eur. J. 2016, 22, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, S.; Pirani, F.; Vecchiocattivi, F. The possible role of penning ionization processes in planetary atmospheres. Atmosphere 2015. [Google Scholar] [CrossRef]
- Alagia, M.; Balucani, N.; Candori, P.; Falcinelli, S.; Pirani, F.; Richter, R.; Rosi, M.; Stranges, S.; Vecchiocattivi, F. Production of ions at high energy and its role in extraterrestrial environments. Rend. Lincei Sci. Fis. Nat. 2013. [Google Scholar] [CrossRef]
- Vuitton, V.; Dutuit, O.; Smith, M.A.; Balucani, N. Chemistry of Titan’s atmosphere. In Titan: Surface, Atmosphere and Magnetosphere; Wodarg, M., Müller-Wodarg, I., Griffith, C.A., Lellouch, E., Cravens, T.E., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 224–284. [Google Scholar]
- Falcinelli, S.; Rosi, M.; Candori, P.; Vecchiocattivi, F.; Farrar, J.M.; Pirani, F.; Balucani, N.; Alagia, M.; Richter, R.; Stranges, S. The escape probability of some ions from Mars and Titan ionospheres. In ICCSA 2014, Part I, Lecture Notes in Computer Science LNCS; Springer: Berlin/Heidelberg, Germany, 2014; Volume 8579, pp. 554–570. [Google Scholar]
- Larsson, M.; Geppert, W.D.; Nyman, G. Ion chemistry in space. Rep. Prog. Phys. 2012, 75, 066901. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Faginas Lago, N.; Laganà, A.; Pirani, F.; Falcinelli, S. A bond-bond portable approach to intermolecular interactions: Simulations for N-methylacetamide and carbon dioxide dimers. In ICCSA 2012, Part I, Lecture Notes in Computer Science LNCS; Springer: Berlin/Heidelberg, Germany, 2012; pp. 387–400. [Google Scholar]
- Pei, L.; Carrascosa, E.; Yang, N.; Falcinelli, S.; Farrar, J.M. Velocity map imaging study of charge-transfer and proton-transfer reactions of CH3 radicals with H3+. J. Phys. Chem. Lett. 2015, 6, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Candori, P.; Falcinelli, S.; Pirani, F.; Tarantelli, F.; Vecchiocattivi, F. Interaction components in the hydrogen halide dications. Chem. Phys. Lett. 2007. [Google Scholar] [CrossRef]
- Sabzyan, H.; Keshavarz, E.; Noorisafa, Z. Diatomic dications and anions. J. Iran. Chem. Soc. 2014, 11, 871. [Google Scholar] [CrossRef]
- Falcinelli, S.; Fernandez-Alonso, F.; Kalogerakis, K.; Zare, R.N. Mass spectrometric detection of alkaline earth monohalide dications. Mol. Phys. 1996, 88, 663–672. [Google Scholar] [CrossRef]
- Tosi, P.; Correale, R.; Lu, W.; Falcinelli, S.; Bassi, D. Production of the molecular dication ArN2+ in the reaction Ar2+ + N2. Phys. Rev. Lett. 1999, 82, 450–452. [Google Scholar] [CrossRef]
- Teixidor, M.M.; Pirani, F.; Candori, P.; Falcinelli, S.; Vecchiocattivi, F. Predicted structure and energetics of HCl2+. Chem. Phys. Lett. 2003, 379, 139–146. [Google Scholar] [CrossRef]
- Alagia, M.; Brunetti, B.G.; Candori, P.; Falcinelli, S.; Teixidor, M.M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Threshold-photoelectron-spectroscopy-coincidence study of the double photoionization of HBr. J. Chem. Phys. 2004, 120, 6985–6991. [Google Scholar] [CrossRef] [PubMed]
- Alagia, M.; Biondini, F.; Brunetti, B.G.; Candori, P.; Falcinelli, S.; Moix, T.M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. The double photoionization of HCl: An ion-electron coincidence study. J. Chem. Phys. 2004, 121, 10508–10512. [Google Scholar] [CrossRef] [PubMed]
- Alagia, M.; Candori, P.; Falcinelli, S.; Pirani, F.; Mundim, M.S.P.; Richter, R.; Rosi, M.; Stranges, S.; Vecchiocattivi, F. Dissociative double photoionization of benzene molecules in the 26–33 eV energy range. Phys. Chem. Chem. Phys. 2011, 13, 8245–8250. [Google Scholar] [CrossRef] [PubMed]
- Price, S.D.; Eland, J.H.D.; Fournier, P.G.; Fournier, J.; Millié, P. Electronic states and decay mechanisms of the N2O2+ dication. J. Chem. Phys. 1988, 88, 1511–1515. [Google Scholar] [CrossRef]
- Slattery, A.E.; Field, T.A.; Ahmad, M.; Hall, R.I.; Lambourne, J.; Penent, F.; Lablanquie, P.; Eland, J.H. Spectroscopy and metastability of CO22+ molecular ions. J. Chem. Phys. 2005. [Google Scholar] [CrossRef] [PubMed]
- Thissen, R.; Delwiche, J.; Robbe, J.M.; Duflot, D.; Flament, J.P.; Eland, J.H.D. Dissociations of the ethyne dication C2H22+. J. Chem. Phys. 1993, 99, 6590–6599. [Google Scholar] [CrossRef]
- Hochlaf, M.; Bennett, F.R.; Chambaud, G.; Rosmus, P. Theoretical study of the electronic states of CO22+. J. Phys. B At. Mol. Opt. Phys. 1998, 31, 2163–2175. [Google Scholar] [CrossRef]
- Sharma, V.; Bapat, B.; Mondal, J.; Hochlaf, M.; Giri, K.; Sathyamurthy, N. Dissociative double ionization of CO2: Dynamics, energy levels, and lifetime. J. Phys. Chem. A 2007, 111, 10205–10211. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. The normal state of the helium molecule-ions He2+ and He2++. J. Chem. Phys. 1933, 1, 56–59. [Google Scholar] [CrossRef]
- Nicolaides, C.A. Energy generation from volcanic ground-states—Application to cold He22+. Chem. Phys. Lett. 1989, 161, 547–553. [Google Scholar] [CrossRef]
- Stauber, P.; Doty, S.D.; van Dishoeck, E.F.; Benz, A.O. X-ray chemistry in the envelopes around young stellar objects. Astron. Astrophys. 2005, 440, 949–966. [Google Scholar] [CrossRef]
- Thissen, R.; Witasse, O.; Dutuit, O.; Wedlund, C.S.; Gronoff, G.; Lilensten, J. Doubly-charged ions in the planetary ionospheres: A review. Phys. Chem. Chem. Phys. 2011, 13, 18264–18287. [Google Scholar] [CrossRef] [PubMed]
- Schio, L.; Li, C.; Monti, S.; Salén, P.; Yatsyna, V.; Feifel, R.; Alagia, M.; Richter, R.; Falcinelli, S.; Stranges, S.; Zhaunerchyk, V. NEXAFS and XPS studies of nitrosyl chloride. Phys. Chem. Chem. Phys. 2015, 17, 9040–9048. [Google Scholar] [CrossRef] [PubMed]
- Alagia, M.; Bodo, E.; Decleva, P.; Falcinelli, S.; Ponzi, A.; Richter, R.; Stranges, S. The soft X-ray absorption spectrum of the allyl free radical. Phys. Chem. Chem. Phys. 2013, 15, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Falcinelli, S.; Rosi, M.; Candori, P.; Vecchiocattivi, F.; Farrar, J.M.; Pirani, F.; Balucani, N.; Alagia, M.; Richter, R.; Stranges, S. Kinetic energy release in molecular dications fragmentation after VUV and EUV ionization and escape from planetary atmospheres. Plan. Space Sci. 2014, 99, 149–157. [Google Scholar] [CrossRef]
- Lilensten, J.; Witasse, O.; Simon, C.; Soldi-Lose, H.; Dutuit, O.; Thissen, R.; Alcaraz, C. Prediction of a N2++ layer in the atmosphere of Titan. Geophys. Res. Lett. 2005, 32, L03203. [Google Scholar] [CrossRef]
- Witasse, O.; Dutuit, O.; Lilensten, J.; Thissen, R.; Zabka, J.; Alcaraz, C.; Blelly, P.L.; Bougher, S.W.; Engel, S.; Andersen, L.H.; et al. Prediction of a CO2++ layer in the atmosphere of Mars. Geophys. Res. Lett. 2002. [Google Scholar] [CrossRef]
- Gronoff, G.; Lilensten, J.; Simon, C.; Witasse, O.; Thissen, R.; Dutuit, O.; Alcaraz, C. Modeling dications in the diurnal ionosphere of Venus. Astron. Astrophys. 2007, 465, 641–645. [Google Scholar] [CrossRef]
- Lilensten, J.; Simon Wedlund, C.; Barthélémy, M.; Thissen, R.; Ehrenreich, D.; Gronoff, G.; Witasse, O. Dications and thermal ions in planetary atmospheric escape. Icarus 2013, 222, 169–187. [Google Scholar] [CrossRef]
- Blyth, R.R.; Delaunay, R.; Zitnik, M.; Krempasky, J.; Krempaska, R.; Slezak, J.; Prince, K.C.; Richter, R.; Vondracek, M.; Camilloni, R.; et al. The high resolution gas phase photoemission beamline, Elettra. J. Electron Spectrosc. Relat. Phenom. 1999, 101–103, 959–964. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Lavollèe, M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Dissociative double photoionization of CO2 molecules in the 36–49 eV energy range: Angular and energy distribution of ion products. Phys. Chem. Chem. Phys. 2010. [Google Scholar] [CrossRef] [PubMed]
- Alagia, M.; Candori, P.; Falcinelli, S.; Lavollée, M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Anisotropy of the angular distribution of fragment ions in dissociative double photoionization of N2O molecules in the 30–50 eV energy range. J. Chem. Phys. 2007. [Google Scholar] [CrossRef] [PubMed]
- Lavollée, M. A new detector for measuring three-dimensional momenta of charged particles in coincidence. Rev. Sci. Instrum. 1990. [Google Scholar] [CrossRef]
- Lundqvist, M.; Baltzer, P.; Edvardsson, D.; Karlsson, L.; Wannberg, B. Novel time of flight instrument for doppler free kinetic energy release spectroscopy. Phys. Rev. Lett. 1995, 75, 1058–1061. [Google Scholar] [CrossRef] [PubMed]
- Field, A.; Eland, J.H.D. Lifetimes of metastable molecular doubly charged ions. Chem. Phys. Lett. 1993, 211, 436–442. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Mundim, K.C.; Mundim, M.S.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Lifetime and kinetic energy release of metastable dications dissociation. Chem. Phys. 2012, 398, 134–141. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Mundim, M.S.P.; Pirani, F.; Richter, R.; Rosi, M.; Stranges, S.; Vecchiocattivi, F. Dissociative double photoionization of singly deuterated benzene molecules in the 26–33 eV energy range. J. Chem. Phys. 2011. [Google Scholar] [CrossRef] [PubMed]
- Alagia, M.; Callegari, C.; Candori, P.; Falcinelli, S.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Angular and energy distribution of fragment ions in dissociative double photoionization of acetylene molecules at 39 eV. J. Chem. Phys. 2012. [Google Scholar] [CrossRef] [PubMed]
- Brooke, T.Y.; Tokunaga, A.T.; Weaver, H.A.; Crovisier, J.; Bockelee-Morvan, D.; Crisp, D. Detection of acetylene in the infrared spectrum of comet Hyakutake. Nature 1996, 383, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Cernicharo, J.; Heras, A.M.; Pardo, J.R.; Tielens, A.G.G.M.; Pardo, J.R.; Herpin, F.; Guélin, M.; Waters, L.B.F.M. Infrared space observatory’s discovery of C4H2, C6H2, and benzene in CRL 618. Astrophys. J. 2001, 546, L123–L126. [Google Scholar] [CrossRef]
- Woods, P.M.; Millar, T.J.; Zijlstra, A.A.; Herbst, E. The synthesis of benzene in the proto-planetary Nebula CRL 618. Astrophys. J. 2002, 574, L167–L170. [Google Scholar] [CrossRef]
- Skouteris, D.; Balucani, N.; Faginas-Lago, N.; Falcinelli, S.; Rosi, M. Dimerization of methanimine and its charged species in the atmosphere of Titan and interstellar/cometary ice analogs. Astron. Astrophys. 2015. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Lavollée, M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Double photoionization of N2O molecules in the 28–40 eV energy range. Chem. Phys. Lett. 2006, 432, 398–402. [Google Scholar] [CrossRef]
- Brunetti, B.; Candori, P.; Falcinelli, S.; Lescop, B.; Liuti, G.; Pirani, F.; Vecchiocattivi, F. Energy dependence of the Penning ionization electron spectrum of Ne*(3P2,0) + Kr. Eur. Phys. J. D 2006, 38, 21–27. [Google Scholar] [CrossRef]
- Cravens, T.E.; Robertson, I.P.; Waite, J.H., Jr.; Yelle, R.V.; Kasprzak, W.T.; Keller, C.N.; Ledvina, S.A.; Niemann, H.B.; Luhmann, J.G.; McNutt, R.L.; et al. Composition of Titan’s ionosphere. Geophys. Res. Lett. 2006, 33, L07105. [Google Scholar] [CrossRef]
- Franceschi, P.; Thissen, R.; Zabka, J.; Roithová, J.; Herman, Z.; Dutuit, O. Internal energy effects in the reactivity of CO22+ doubly charged molecular ions with CO2 and CO. Int. J. Mass Spectrom. 2003, 228, 507–516. [Google Scholar] [CrossRef]
- Alagia, M.; Candori, P.; Falcinelli, S.; Lavollée, M.; Pirani, F.; Richter, R.; Stranges, S.; Vecchiocattivi, F. Double photoionization of CO2 molecules in the 34–50 eV Energy range. J. Phys. Chem. A 2009, 113, 14755–14759. [Google Scholar] [CrossRef] [PubMed]




| Ionic Species | Measured Range of KER Distributions (eV) | Typical Escape Energy (eV) for Various Ions in the Atmosphere (at the Exobase) of Some Planets of the Solar System [28] | |||
|---|---|---|---|---|---|
| Earth | Venus | Mars | Titan | ||
| H+ | 2.8 ÷ 6.0 (a) | 0.62 | 0.53 | 0.13 | 0.02 |
| C+ | 1.7 ÷ 3.3 (b) | 7.4 | 6.4 | 1.5 | 0.28 |
| CH+ | 1.3 ÷ 3.2 (c) | 8.0 | 6.9 | 1.6 | 0.30 |
| CH2+ | 1.6 ÷ 2.9 (b) | 8.6 | 7.5 | 1.8 | 0.32 |
| N+ | 2.3 ÷ 5.2 (d) | 8.6 | 7.5 | 1.8 | 0.32 |
| O+ | 1.0 ÷ 5.2 (e) 2.2 ÷ 3.8 (f) | 9.8 | 8.6 | 2.0 | 0.37 |
| C2H+ | 0.1 ÷ 0.4 (a) | 15.4 | 13.3 | 3.1 | 0.58 |
| CO+ | 0.4 ÷ 2.6 (f) | 17.3 | 14.9 | 3.5 | 0.65 |
| N2+ | 0.4 ÷ 2.9 (e) | 17.3 | 14.9 | 3.5 | 0.65 |
| NO+ | 1.0 ÷ 2.9 (d) | 18.5 | 16.0 | 3.75 | 0.70 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falcinelli, S.; Pirani, F.; Alagia, M.; Schio, L.; Richter, R.; Stranges, S.; Balucani, N.; Vecchiocattivi, F. Molecular Dications in Planetary Atmospheric Escape. Atmosphere 2016, 7, 112. https://doi.org/10.3390/atmos7090112
Falcinelli S, Pirani F, Alagia M, Schio L, Richter R, Stranges S, Balucani N, Vecchiocattivi F. Molecular Dications in Planetary Atmospheric Escape. Atmosphere. 2016; 7(9):112. https://doi.org/10.3390/atmos7090112
Chicago/Turabian StyleFalcinelli, Stefano, Fernando Pirani, Michele Alagia, Luca Schio, Robert Richter, Stefano Stranges, Nadia Balucani, and Franco Vecchiocattivi. 2016. "Molecular Dications in Planetary Atmospheric Escape" Atmosphere 7, no. 9: 112. https://doi.org/10.3390/atmos7090112