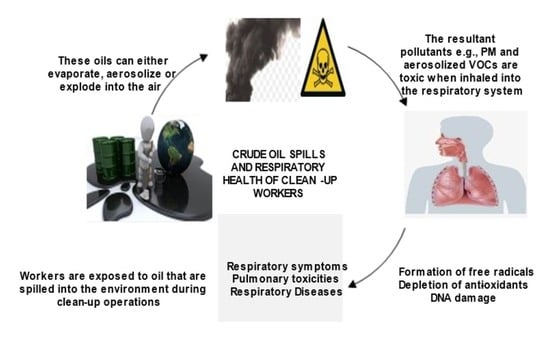
Crude Oil Spills and Respiratory Health of Clean-Up Workers: A Systematic Review of Literature
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Inclusion Criteria and Exclusion Criteria
2.4. Quality of Evidence
2.5. Data Extraction and Data Synthesis
3. Results
3.1. Search Results
3.2. Methodological Analysis
3.2.1. Study Design
3.2.2. Place of Research
3.3. Analysis of Results
3.4. Crude Oil Spills
3.4.1. Tasman Spirit Oil Spill (TS Spill)
3.4.2. Deepwater Horizon Oil Spill (DWH Spill)
3.4.3. Hebei Spirit Oil Spill (HS Spill)
3.4.4. Prestige Oil Spill
3.5. Crude Oil Spills and Respiratory Health
3.5.1. Tasman Oil Spill and Respiratory Health of Clean-Up Workers
3.5.2. DWH Oil Spill and Respiratory Health of Clean-Up Workers
3.5.3. Hebei Spirit Oil Spill and Respiratory Health of Clean-Up Workers
3.5.4. Prestige Oil Spill and Respiratory Health of Clean-Up Workers
4. Discussion
5. Challenges and Recommendation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CS | Cross-sectional Study |
| CC | Case Control |
| PS | Prospective Study |
| BTEX | Benzene, Toluene, Ethylbenzene, Xylene |
| BTEX-H | Benzene, Toluene, Ethylbenzene, o-, m-, and p-Xylenes, and n-Hexane |
| VOCs | Volatile Organic Compounds |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| SO2, | Sulphur dioxide |
| NO2 | Nitrogen dioxide |
| PM2.5 | Fine Particulate Matter |
| PM0.1 | Ultrafine Particulate Matter |
| WHO | World Health Organization |
| DALYS | Disability Adjusted Life Years |
| COPD | Chronic Obstructive Pulmonary Disease |
| PRISMA | Preferred Reporting Items for Systematic Review and Meta-Analysis |
| IAP | Indoor Air Pollution |
| AAP | Ambient Air Pollution |
| FEV1 | Forced Expiratory Volume in one second |
| MBPT | Methacholine Bronchial Provocation Test |
| FEF | Forced Expiratory Flow |
| MVV | Maximum Voluntary Ventilation |
| DWH | Deep Water Horizon |
| OSRC | Oil Spill Response and Clean-up Workers |
| HS | Hebei Spirit |
| TS | Tasman Spirit |
| DNA | Deoxyribonucleic acid |
| ROS | Reactive Oxygen Species |
| MD | Mean Difference |
| RD | Risk Difference |
| SES | Socio-economic status |
| KL | Kilolitres |
| SHS | Secondhand smoking |
| RH | Respiratory health |
| aHR | Adjusted Hazard Ratio |
References
- Chen, J.; Zhang, W.; Wan, Z.; Li, S.; Huang, T.; Fei, Y. Oil Spills from Global Tankers: Status Review and Future Governance. J. Cleaner Prod. 2019, 227, 20–32. [Google Scholar] [CrossRef]
- Obida, C.B.; Blackburn, G.A.; Whyatt, J.D.; Semple, K.T. Quantifying the Exposure of Humans and The Environment To Oil Pollution In The Niger Delta Using Advanced Geostatistical Techniques. Environ. Int. 2018, 111, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemzero, D.A.; Igbal, N.; Iqbal, S.; Mohsin, M.; Chukwuma, N.J.; Shah, B.A. Assessing the Perceived Impact of Exploration and Production Of Hydrocarbons On Households Perspective Of Environmental Regulation In Ghana. Environ. Sci. Pollut. Res. 2021, 28, 5359–5371. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Impact of Total Petroleum Hydrocarbons on Human Health. In Total Petroleum Hydrocarbons; Springer: Cham, Switzerland, 2020; pp. 139–165. [Google Scholar]
- Wilkes, H.; Jarling, R.; Schwarzbauer, J. Hydrocarbons and Lipids: An Introduction to Structure, Physiochemical Properties, And Natural Occurrence. In Hydrocarbons, Oils and Lipids: Diversoty, Origin, Chemistry and Fate; Springer: Cham, Switzerland, 2020; pp. 3–48. [Google Scholar]
- Major, D.N.; Wang, H. How Public Health Impact Is Addressed: A Retrospective View on Three Different Oil Spills. Toxicol. Environ. Chem. 2012, 94, 442–467. [Google Scholar] [CrossRef]
- Rajabi, H.; Mosleh, M.H.; Mandal, P.; Lea-Langton, A.; Sedighi, M. Emissions of Volatile Organic Compounds From Crude Oil Processing-Global Emissions Inventory And Environmental Release. Sci. Total Environ. 2020, 727, 138654. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.; Engel, L.S.; Olaiya, N.; Wang, L.; Barrett, J.; Weems, L.; Schwartz, E.G.; Rusiecki, J.A. The Deepwater Horizon Oil Spill Coast Guard Cohort Study: A Cross-Sectional Study Of Acute Respiratory Health Symptoms. Environ. Res. 2018, 162, 196–202. [Google Scholar] [CrossRef]
- Ramírez, N.; Cuadras, A.; Rovira, E.; Borrull, F.; Marcé, R.M. Chronic Risk Assessment of Exposure To Volatile Organic Compounds In The Atmosphere Near The Largest Mediterranean Industrial Site. Environ. Int. 2012, 39, 200–209. [Google Scholar] [CrossRef]
- Ifegwu, O.C.; Anyakora, C. Polycyclic Aromatic Hydrocarbons: Part I. Exposure. Adv. Clin. Chem. 2015, 72, 277–304. [Google Scholar]
- Nnaemeka, A.N. Environmental Pollution and Associated Health Hazards to Host Communities (Case Study: Niger Delta Region of Nigeria). Cent. Asian J. Environ. Sci. Technol. Innov. 2020, 1, 30–42. [Google Scholar]
- Zhang, B.; Matchinski, E.J.; Chen, B.; Ye, X.; Jing, L.; Lee, K. Marine Oil Spills—Oil Pollution, Sources and Effects. In World Seas: An Environmental Evaluation; Academic Press: Cambridge, MA, USA, 2019; pp. 391–406. [Google Scholar]
- Clarke, K.C.; Hemphill, J.J. The Santa Barbara Sil spill. A Retrospective. Yearb. Assoc. Pac. Coast Geogr. 2002, 64, 157–162. [Google Scholar]
- Law, R.J. The Torrey Canyon Oil Spill, 1967. In Oil Spill Science and Technology; Gulf Professional Publishing: Amsterdam, The Netherlands, 2011; pp. 1103–1106. [Google Scholar]
- Nriagu, J.; Udofia, E.A.; Ekong, I.; Ebuk, G. Health risks associated with oil pollution in the Niger Delta, Nigeria. Int. J. Environ. Res. Public Health 2016, 13, 346. [Google Scholar] [CrossRef] [Green Version]
- Vallero, D. Unraveling Environmental Disaster; Newnes: Oxford, UK, 2012. [Google Scholar]
- Mishra, A.K.; Kumar, G.S. Weathering of Oil Spill: Modeling and Analysis. Aquat. Procedia 2015, 4, 435–442. [Google Scholar] [CrossRef]
- Yang, Z.; Shah, K.; Laforest, S.; Hollebone, B.P.; Situ, J.; Crevier, C.; Lambert, P.; Brown, C.E.; Yang, C. Occurrence And Weathering Of Petroleum Hydrocarbons Deposited On The Shoreline Of The North Saskatchewan River From the 2016 Husky Oil Spill. Environ. Pollut. 2020, 258, 113769. [Google Scholar] [CrossRef]
- Singh, H.; Bhardwaj, N.; Arya, S.K.; Khatri, M. Environmental Impacts of Oil Spills and Their Remediation By Magnetic Nanomaterials. Environ. Nanotechnol. Monit. Manag. 2020, 14, 1003052020. [Google Scholar] [CrossRef]
- Muthukumar, B.; Al Salhi, M.S.; Narenkumar, J.; Devanesan, S.; Rao, T.N.; Kim, W.; Rajasekar, A. Characterization of Two Novel Strains of Pseudomonas Aeuruginosa On Biodegradation Of Crude Oil And Its Enzyme Activities. Environ. Pollut. 2022, 304, 119223. [Google Scholar] [CrossRef]
- Passow, U.; Overton, E.B. The Complexity of Spills: The Fate of The Deepwater Horizon Oil. Annu. Rev. Mar. Sci. 2021, 13, 109–136. [Google Scholar] [CrossRef]
- Afshar-Mohajer, N.; Fox, M.A.; Koehler, K. The Human Health Risk Estimation of Inhaled Oil Spill Emissions with And Without Adding Dispersant. Sci. Total Environ. 2019, 654, 924–932. [Google Scholar] [CrossRef]
- Farrington, J.W. Oil Pollution in The Marine Environment II: Fates and Effects of Oil Spills. Environ. Sci. Policy Sustain. Dev. 2014, 56, 16–31. [Google Scholar] [CrossRef]
- Beedasy, J.; Petkova, E.P.; Lackner, S.; Sury, J. Gulf Coast Parents Speak: Children’s Health in The Aftermath Of The Deepwater Horizon Oil Spill. Environ. Hazards 2021, 20, 248–263. [Google Scholar] [CrossRef]
- WHO. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 12 February 2022).
- WHO. Ambient (Outdoor) Air Pollution. Available online: https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health (accessed on 17 December 2021).
- Simoni, M.; Baldacci, S.; Maio, S.; Cerrai, S.; Sarno, G.; Viegi, G. Adverse Effects of Outdoor Pollution in The Elderly. J. Thorac. Dis. 2015, 7, 34. [Google Scholar]
- Elum, Z.A.; Mopipi, K.; Henri-Ukoha, A. Oil Exploration and Its Socioeconomic Effects on The Niger Delta Region of Nigeria. Environ. Sci. Pollut. Res. 2016, 23, 12880–12889. [Google Scholar] [CrossRef] [PubMed]
- Santos, U.; Correa, L.; Cahtkin, J.M. External Environmental Pollution as A Risk Factor For Asthma. Clin. Rev. Allergy Immunol. 2021, 62, 72–89. [Google Scholar]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; Moradi, M. Prevalence and Attributable Health Burden Of Chronic Respiratory Diseases, 1990–2017: A Systematic Analysis For The Global Burden Of Disease Study 2017. Lancet Respir. Med. 2020, 8, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Societies, F.o.I.R. The Global Impact of Respiratory Disease—Second Edition; European Respiratory Society: Brussels, Belgium, 2017. [Google Scholar]
- Chen, R.; Hu, B.; Liu, Y.; Xu, J.; Yang, G.; Xu, D.; Chen, C. Beyond PM2. 5: The Role of Ultrafine Particles On Adverse Health Effects Of Air Pollution. Biochim. Biophys. Acta BBA-Gen. Subj. 2016, 1860, 2844–2855. [Google Scholar] [CrossRef]
- Kim, B.J.; Hong, S.J. Ambient Air Pollution and Allergic Diseases in Children. Korean J. Pediatr. 2012, 55, 185. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, R.B.; Gardner, D.E. Respiratory Tract Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Laffon, B.; Pásaro, E.; Valdiglesias, V. Effects of Exposure to Oil Spills on Human Health: Updated Review. J. Toxicol. Environ. Health Part B 2016, 19, 105–128. [Google Scholar] [CrossRef]
- Kuran, C.H.A.; Morsut, C.; Kruke, B.I.; Krüger, M.; Segnestam, L.; Orru, K.; Torpan, S.; Keränen, J. Vulnerability and Vulnerable Groups From An Intersectionality Perspective. Int. J. Disaster Risk Reduct. 2020, 50, 101826. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Stewart, L.A. Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kansagara, D.; O’Neil, M.; Nugent, S.; Freeman, M.; Low, A.; Kondo, K.; Elven, C.; Zakher, B.; Motu’apuaka, M.; Morasco, B.J.; et al. Benefits and Harms of Cannabis in Chronic Pain or Post-Traumatic Stress Disorder: A Systematic Review; Department of Veterans Affairs: Washington, DC, USA, 2018. [Google Scholar]
- Meo, S.A.; Al-Drees, A.M.; Meo, I.M.; Al-Saadi, M.M.; Azeem, M.A. Lung Function in Subjects Exposed to Crude Oil Spill Into Sea Water. Mar. Pollut. Bull. 2008, 56, 88–94. [Google Scholar] [CrossRef]
- Meo, S.A.; Al-Drees, A.M.; Rasheed, S.; Meo, I.M.; Al-Saadi, M.M.; Ghani, H.A.; Alkandari, J.R. Health Complaints Among Subjects Involved in Oil Cleanup Operations During Oil Spillage from A Greek Tanker “Tasman Spirit”. Int. J. Occup. Med. Environ. Health 2009, 22, 143. [Google Scholar] [CrossRef]
- Meo, S.A.; Al-Drees, A.M.; Rasheed, S.; Meo, I.M.; Khan, M.M.; Al-Saadi, M.M.; Alkandari, J.R. Effect of Duration of Exposure to Polluted Air Environment on Lung Function in Subjects Exposed to Crude Oil Spill Into Sea Water. Int. J. Occup. Med. Environ. Health 2009, 22, 35–41. [Google Scholar] [CrossRef]
- D’Andrea, M.A.; Reddy, G.K. The Development of Long-Term Adverse Health Effects in Oil Spill Cleanup Workers Of The Deepwater Horizon Offshore Drilling Rig Disaster. Front. Public Health 2018, 6, 117. [Google Scholar] [CrossRef] [Green Version]
- Gam, K.B.; Engel, L.S.; Kwok, R.K.; Curry, M.D.; Stewart, P.A.; Stenzel, M.R.; McGrath, J.A.; Jackson, B., 2nd; Litchveld, M.Y.; Sandler, D.P.; et al. Association Between Deepwater Horizon Oil Spill Response and Cleanup Work Experiences And Lung Function. Environ. Int. 2018, 121, 695–702. [Google Scholar] [CrossRef]
- Gam, K.B.; Kwok, R.K.; Engel, L.S.; Curry, M.D.; Stewart, P.A.; Stenzel, M.R.; McGrath, J.A.; Jackson, B., 2nd; Jensen, R.L.; Keil, A.P.; et al. Lung Function In Oil Spill Response Workers 1–3 years After The Deepwater Horizon Disaster. Epidemiology 2018, 29, 315. [Google Scholar] [CrossRef]
- Gam, K.B.; Kwok, R.K.; Engel, L.S.; Curry, M.D.; Stewart, P.A.; Stenzel, M.R.; McGrath, J.A.; Jackson, B., 2nd; Litchveld, M.Y.; Sandler, D.P. Exposure To Oil Spill Chemicals And Lung Function In Deepwater Horizon Disaster Response Workers. J. Occup. Environ. Med. 2018, 60, e312. [Google Scholar] [CrossRef]
- Lawrence, K.G.; Keil, A.P.; Garantziotis, S.; Umbach, D.M.; Stewart, P.A.; Stenzel, M.R.; McGrath, J.A.; Jackson, B., 2nd; Kwok, R.K.; Sandler, D.P. Lung function in oil spill responders 4–6 years after the Deepwater Horizon disaster. J. Toxicol. Environ. Health Part A 2020, 83, 233–248. [Google Scholar] [CrossRef]
- Lawrence, K.G.; Niehoff, N.M.; Keil, A.P.; Jackson II, W.B.; Christenbury, K.; Stewart, P.A.; Sandler, D.P. Associations Between Airborne Crude Oil Chemicals and Symptom-Based Asthma. Environ. Int. 2022, 167, 107433. [Google Scholar] [CrossRef]
- Chen, D.; Lawrence, K.G.; Pratt, G.C.; Stenzel, M.R.; Stewart, P.A.; Groth, C.P.; Banerjee, S.; Christenbury, K.; Curry, M.D.; Sandler, D.P. Fine Particulate Matter And Lung Function Among Burning-Exposed Deepwater Horizon Oil Spill Workers. Environ. Health Perspect. 2022, 130, 027001. [Google Scholar] [CrossRef] [PubMed]
- Rusiecki, J.; Alexander, M.; Schwartz, E.G.; Wang, L.; Weems, L.; Barrett, J.; Christenbury, K.; Johndrow, D.; Funk, R.H.; Engel, L.S. The Deepwater Horizon Oil Spill Coast Guard Cohort Study. Occup. Environ. Med. 2018, 75, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Andrea, M.A.; Reddy, G.K. Health Consequences Among Subjects Involved in Gulf Oil Spill Clean-up Activities. Am. J. Med. 2013, 126, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Rusiecki, J.A.; Denic-Roberts, H.; Thomas, D.L.; Collen, J.; Barrett, J.; Christenbury, K.; Engel, L.S. Incidence of Chronic Respiratory Conditions Among Oil Spill Responders: Five Years of Follow-up In The Deepwater Horizon Oil Spill Coast Guard Cohort Study. Environ. Res. 2022, 203, 1118242022. [Google Scholar] [CrossRef]
- Gwack, J.; Lee, J.H.; Kang, Y.A.; Chang, K.J.; Lee, M.S.; Hong, J.Y. Acute Health Effects Among Military Personnel Participating in The Cleanup of The Hebei Spirit Oil Spill, 2007, In Taean County, Korea. Osong Public Health Res. Perspect. 2012, 3, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Sim, M.S.; Jo, I.J.; Song, H.G. Acute Health Problems Related to The Operation Mounted to Clean The Hebei Spirit Oil Spill In Taean, Korea. Mar. Pollut. Bull. 2010, 60, 51–57. [Google Scholar] [CrossRef]
- Rodríguez-Trigo, G.; Zock, J.-P.; Pozo-Rodríguez, F.; Gómez, F.P.; Monyarch, G.; Bouso, L.; Coll, M.D.; Verea, H.; Antó, J.M. Health Changes In Fishermen 2 Years After Clean-up Of The Prestige Oil Spill. Ann. Intern. Med. 2010, 153, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Zock, J.P.; Rodríguez-Trigo, G.; Rodríguez-Rodríguez, E.; Espinosa, A.; Pozo-Rodríguez, F.; Gómez, F.; Castaño-Vinyals, G.; Antó, J.M.; Barberà, J.A. Persistent Respiratory Symptoms In Clean-up Workers 5 Years After The Prestige Oil Spill. Occup. Environ. Med. 2012, 69, 508–513. [Google Scholar] [CrossRef]
- Suárez, B.; Lope, V.; Pérez-Gómez, B.; Aragonés, N.; Rodríguez-Artalejo, F.; Marqués, F.; Guzmán, A.; Viloria, L.J.; Carrasco, J.M.; Pollán, M. Acute Health Problems Among Subjects Involved in The Cleanup Operation Following the Prestige Oil Spill In Asturias And Cantabria (Spain). Environ. Res. 2005, 99, 413–424. [Google Scholar] [CrossRef]
- Zock, J.P.; Rodríguez-Trigo, G.; Rodríguez-Rodríguez, E.; Souto-Alonso, A.; Espinosa, A.; Pozo-Rodríguez, F.; Gómez, F.P.; Fuster, C.; Castaño-Vinyals, G.; Barberà, J.A.; et al. Evaluation of The Persistence of Functional and Biological Respiratory Health Effects in Clean-up Workers 6 Years After The Prestige Oil Spill. Environ. Int. 2014, 62, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Ephraim-Emmanuel, B.C.; Ordinioha, B. Exposure and Public Health Effects Of Polycyclic Aromatic Hydrocarbon Compounds In Sub-Saharan Africa: A Systematic Review. Int. J. Toxicol. 2021, 40, 250–269. [Google Scholar] [CrossRef]
- Katoto, P.D.; Byamungu, L.; Brand, A.S.; Mokaya, J.; Strijdom, H.; Goswami, N.; De Boever, K.; Nawrot, T.S.; Nemery, B. Ambient Air Pollution And Health In Sub-Saharan Africa: Current Evidence, Perspectives And A Call To Action. Environ. Res. 2019, 173, 174–188. [Google Scholar] [CrossRef]
- Bede-Ojimadu, O.; Orisakwe, O.E. Exposure to Wood Smoke and Associated Health Effects In Sub-Saharan Africa: A Systematic Review. Ann. Glob. Health 2020, 86, 32. [Google Scholar] [CrossRef] [Green Version]
- Mathieu-Nolf, M. Poisons in The Air: A Cause of Chronic Disease in Children. J. Toxicol. Clin. Toxicol. 2002, 40, 483–491. [Google Scholar] [CrossRef] [PubMed]
- De Chesnay, M. Vulnerable Population: Vulnerable People. Caring Vulnerable 2008, 2, 1–14. [Google Scholar]
- Noh, S.R.; Kim, J.A.; Cheong, H.K.; Ha, M.; Jee, Y.K.; Park, M.S.; Choi, K.-W.; Kim, H.; Cho, S.-I.; Paek, D.; et al. Hebei Spirit Oil Spill and Its Long-term Effect On Children’s Asthma Symptoms. Environ. Pollut. 2019, 248, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Choi, K.H.; Lee, S.H.; Hur, J.I.; Noh, S.R.; Jeong, W.C.; Cheong, H.-K.; Ha, M. Health Effect Research On Hebei Spirit Oil Spill (HEROS) In Korea: A Cohort Profile. BMJ Open 2019, 9, e026740. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Sanchez, C.E.R.M. J Solli P Longitudinal Studies. J. Thorac. Dis. 2015, 7, E537. [Google Scholar]

| S/N | Name of Spills | Study Location | Year of Spill | Quantity Spilled | Reference |
|---|---|---|---|---|---|
| 1. | Tasman Spirit Oil Spill | Coastal areas of Karachi, Pakistan | 2003 | 35,298 KL (30,000 tons) | [39,40,41] |
| 2. | Deepwater Horizon Oil Spill | Gulf of Mexico | 2010 | About 757,082 KL (200 million gallons or 4,761,905 barrels or 649,645 metric tons) | [8,42,43,44,45,46,47,48,49,50,51]. |
| 3. | Hebei Spirit Oil Spill | Taean area, Korea | 2007 | 12,942.6 KL (an estimated 11,000 tons) | [52,53]. |
| 4. | Prestige Oil Spill | Asturia and Cantabria, Spain | 2002 | 77,123.8 KL (67,000 tons) of bunkered oil | [54,55,56,57]. |
| S/N | Reference; Study Location and Study Period | Study Design, Study Population, Sample Size | Oil Spill | Methods of Assessing Respiratory Effects | Study Findings | Adjustment for Confounding Factors |
|---|---|---|---|---|---|---|
| 1. | [39]; Karachi, Pakistan; August 2003–2004 | a CS with a 1-year follow-up study; Clean-up workers (n = 20 males) vs. matched controls (clerical staff, shopkeepers, and salesmen) (n = 31 males) | Tasman Spirit oil | Spirometry; Detailed interview, Questionnaire | A remarkable decrease in FVC, FEV1, (FEF25–75%), and MVV in those exposed to polluted air in comparison to matched controls. | Age, height, body mass, socio-economic status (SES), cigarette smokers, respondents with industrial exposure to smoke or dust |
| 2. | [40]; Coastal areas of Karachi, Pakistan; July 2003–December 2004 | a CC; Clean-up workers; (healthy men, n = 50) vs. controls (clerical staff, shopkeepers, and salesmen) (n = 50) | Tasman Spirit oil | Standardized Questionnaire, Detailed interview | Greater prevalence of cough, rhinorrhoea, sore throat, malaise, dyspnoea, chest tightness, phlegm, and wheeze, which were 40%, 36%, 30%, 18%, 14%, 10% and 6%, respectively, compared to controls. The odds of sore throat; cough and runny nose ((OR, 95% CI): 6.09, 2.0–22.8; p < 0.006 *); (9.60, 2.61–35.22; p < 0.0002 *); and (14.0, 3.0–62.0; p < 0.0001 *) were markedly higher among the clean-up workers than the controls. | Age, sex, SES, respondents addicted to drugs, cigarette smokers, exposed to smoke and dust from any industry, and working at petrol pumps and gas stations |
| 3. | [41]; Coastal areas of Karachi, Pakistan; July 2003–December 2004 | Comparative study; Clean-up workers; Healthy, non-smoking male workers, n = 31 vs. clerical staff, shopkeepers and salesmen, n = 31 | Tasman oil | Standardized Questionnaire; Spirometry | Being exposed to the spill had a decrease in FVC, FEV1, FEF25–75%, and MVV that is statistically significant (p = 0.001 *; 0.001 *; 0.002 *; and 0.001 *). Exposure to these pollutants for less than 8 days had a significant difference in only FVC (p = 0.001); for 8–15 days revealed a significant difference for FVC (p = 0.05); and for greater than 15 days, revealed a significant decrease in for FVC, FEV1, FEF25–75%, and MVV in the exposed group relative to their controls (p = 0.001 *; 0.001 *; 0.02 *; and 0.002 *). | Subjects that smoke, SES, occupational exposure history, Age, Height, Weight |
| 4. | [49]; Gulf of Mexico; (20 April–17 December 2010 | a PS, CS; DWH oil spill responder (US Coast Guard personnel); (n = 8700) and non-responders (n = 44,800) | DWH oil spill following oil rig explosion | Objective health data, Survey data | Increased PRs were statistically significant and this increased with exposure for all three symptoms: cough (PR = 1.6–1.8); wheeze (PR = 2.1–2.3); dyspnoea (PR = 1.8–2.3). Elevated risk for chronic respiratory diseases (RR = 1.3; 95% CI: 1.0 to 2.0), with asthma inclusive (RR = 2.0; 95% CI 1.0 to 3.2); increased RRs were also found for COPD (RR = 1.4; 95% CI: 0.97 to 1.90) 2.5 years after exposure. | - |
| 5. | [51]; Gulf of Mexico; 1 October 2007–30 September 2015 | Prospective analysis; Prospective follow-up; (US Coast Guard personnel) responders vs. non-responders; n = 45,190 | DWH oil spill following oil rig explosion | Medical encounter data | Responder/non-responder: Weak elevated adjusted hazard ratios (aHRs) Responder comparisons: Stronger risks with exposure to crude oil. Exposure through inhaling the pollutants: Elevated risks for all sinusitis, unidentified long-term sinusitis, COPD and other related health conditions, and dyspnea and respiratory abnormalities [(aHR; 95% CI) (1.5; 1.1–2.1), (1.6; 1.1–2.2), (1.4; 1.0–2.1), (1.3; 1.0–1.7)]; elevated risk for diseases categorized as asthma and reactive airway diseases, including the specific condition, asthma, the symptom, dyspnoea, and the general categorization of long-term respiratory conditions [(aHR;95% CI): (1.2; 1.0–1.4), (1.4; 1.0–2.3), (1.5; 1.0–2.5), and (1.2; 1.0–1.4). Positive associations between exposure to both crude oil and dispersant and an increased risk for shortness of breath (HR = 2.0; 95% CI, 1.0–5.0). | Smokers |
| 6. | [50]; Gulf of Mexico; January 2010 and November 2012 | a CS; Clean-up workers; n = 247 subjects (exposed vs. non-exposed, 117 vs. 130) | DWH oil spill following oil rig explosion | Self-reported data on somatic symptoms | Some respiratory somatic symptoms include headache 77%, dyspnoea 71%, dermatitis, chronic cough 52%, chest pain 38%. | - |
| 7. | [42]; Gulf of Mexico; | Follow-up study after 7-years exposure; Clean-up workers; exposed = 44, non-exposed = 44 | DWH oil spill following oil rig explosion | Medical records and charts | After 7-years exposure (long-term exposure), chronic rhinosinusitis and reactive airway dysfunction syndrome developed. | - |
| 8. | [8]; Gulf of Mexico; 20 April 2010–15 July 2010 vs. after 15 July 2010–30 September | a CS; United States Coast Guard personnel; (n = 4855) | DWH oil spill following oil rig explosion | Self-reported data; Questionnaire | Cough (19.4%); dyspneoa (6.0%); wheeze (4.0%) Adjusted analyses showed elevated PRs for cough (PR = 1.9), dyspnoea (PR = 2.6), wheeze (PR = 2.7) for any exposure to oil. A sub-analysis was performed comparing those responders who were exposed to oil alone, those exposed to a combination of oil and oil dispersant, and those who were not exposed. Exposure to oil alone had raised PRs for cough, dyspnoea, and wheeze [(PR;95% CI): (1.7; 1.4–2.0), dyspnoea (2.0; 1.2–3.0), and wheeze (2.0; 1.4–3.6). Greater PRs recorded with respect to cough during exposure to a combination of oil and oil dispersant (PR: 2.7; 95% CI: 2.3–3.2), dyspnoea (PR: 5.0; 95% CI: 3.3–7.0), wheeze (PR: 5.1; 95% CI: 3.2–8.0). | Duration of deployment |
| 9. | [43]; Gulf of Mexico; May 2011–May 2013 | a PC Case analysis; Clean-up workers; n = 4806 | DWH oil spill following oil rig explosion | Spirometry; Questionnaire | Higher FEV1 (MD: 30 mL; 95% CI: −3, 64), and FVC (MD: 30 mL, 95% CI: −9, 69) values among those that smelled chemicals than unexposed workers. A significantly lower FEV1 (MD: −70 mL, 95% CI: −105, −30), FVC (MD: −60 mL, 95% CI: −97, −15) and FEV1/FVC (MD: −0.60%, 95% CI: −1.0, −0.2) among clean-up workers with exposure due to oily flora/fauna or dead animal recovery jobs compared to unexposed [FEV1 (MD: −50 mL, 95% CI: −80, −20); FVC (MD: −45 mL, 95% CI: −80, −9); FEV1/FVC ratio (MD: −0.4%, 95% CI: −0.80, −0.07)]. | Maximum ordinal THC exposure levels, exposure to blazing oil/gas and dispersant; age in years, height, height-squared, body mass, male/female, origin, race; diabetes and pulmonary disease diagnosis before the spill; salary, education, employment, subjects with a history of oil company experience and clean-up operations; residing close to coastal regions, smokers and secondhand smoking (SHS) history |
| 10. | [44]; Gulf of Mexico; May 2011–May 2013 | a PC; Clean-up workers; n = 7780 | DWH oil spill following oil rig explosion | Spirometry; Questionnaire | Some decrease in FEV1 (β: −70 mL, 95% CI: −130, −10) in decontamination workers compared to support workers. Exposed workers to flaming oil/gas had decrements in their pulmonary function with respect to the unexposed workers: FEV1 and FEV1/FVC [(β;95% CI): −180 mL; −320, −50) and (β: −2.0%; −3.5, −0.4), and a raised risk of having a FEV1/FVC in the minimum tertile (PR: 1.4, 95% CI: 1.0–2.0) | Age; male/female; race; educational attainment; employment; past medical history of lung disease and diabetes; and work-related exposure history, residential proximity, exposure to secondhand smoke |
| 11. | [45]; Gulf of Mexico; | a PC; Clean-up workers; n = 6288 workers | DWH oil spill following oil rig explosion | Questionnaire; Spirometry | The lung function in general was not different by THC exposure levels among workers who partook in clean-up activities, who were highly exposed compared to the less exposed, hence no association was noticed between THC exposure and pulmonary function of workers that participated in clean-up operations 1 to 3 years after the spill | Age; gender; race; educational attainment; employment; past medical history of lung disease and diabetes; and work-related exposure history; residential proximity, exposure to secondhand smoke |
| 12. | [48]; Gulf of Mexico; 15 May to 15 July 2010 | a PC; Clean-up workers (exposed to burning and referent group); n = 2320 (n = 518 and n = 1798) | DWH oil spill following oil rig explosion | Spirometry; Questionnaire | Exposure–response trends showed significant associations between elevated total daily greatest PM2.5 exposure with reduced FEV1 (p-trend = 0.04), FEV1/FVC (p-trend = 0.01). Compared with less-exposed workers, those with greater total exposures had decrements in FEV1 [−167.0 mL, 95% CI: −337.0, 4.0] and FEV1/FVC (−2.0, 95% CI: −4.0, 0.2). A significant observation was made between average daily greatest exposure and FEV1 (p-trend = 0.02) and significantly lower FEV1 (−228.0 mL, 95% CI: −431.0, −25.0) among the workers who never smoked in the high-exposure group, as well as a lower FVC among never-smokers with higher average and cumulative daily maximum exposures. A statistically significant trend for the association between cumulative daily maximum exposure and FEV1/FVC (p-trend = 0.01) accompanied by insignificantly lower FEV1/FVC in the high-exposure group (−3.0%, 95% CI: −6.0, 0.1) in the never-smokers sub-group | Sex, race, highest educational attainment; employment; cigarette smoking status; past medical history of lung disease and diabetes; and occupational exposure history, residential proximity to spill, exposure to secondhand smoke |
| 13. | [46]; Gulf of Mexico; Between August 2014 and June 2016 | a PC; OSRC; n = 1840 (Worker vs. Non-worker: 270 vs. 1570) | DWH oil spill following oil rig explosion | Questionnaire; Spirometry | A total of 4–6 years after exposure, clean-up responders with THC exposure 1.0–3.0 ppm and ≥3.0 ppm had higher FEV1 when compared to responders with ≤0.3 ppm (β: 110 mL, 95% CI: 20, 200), and (β: 120 mL, 95% CI: 5–230). Decrease in lung function was no longer evident after 4–6 years. Greatest exposures had the greatest improvement in their respiratory health. | Age; age-squared; height; height-squared; weight; female/male; Hispanic ethnicity; race; past medical history of diabetes or lung disease; educational level; occupation; previous oil company involvement; previous oil spill response history; smoking status |
| 14. | [47]; Gulf of Mexico; | a PC; Clean-up workers; n = about 24,610 (19,020 workers; 5590 nonworkers) | DWH oil spill following oil rig explosion | Self-reported data using Questionnaire | Workers who participated in the clean-up activities had greater risks of developing asthma than non-workers (RR: 1.6, 95% CI: 1.0–2.0). Increased risk with exposure to higher THC levels (p < 0.0001 *). Risk of developing asthma was elevated with an elevated exposure to individual BTEX-H chemicals and the chemical mixture RR: 1.5; 95% CI: 1.4–1.6. For physician-diagnosed asthma, associations were less apparent. | Age; male/female; Hispanic ethnicity; race; past medical history of diabetes or lung disease; highest educational attainment; employment status; history of prior oil company experience; history of oil spill cleanup operations; smoking status |
| 15. | [52]; Taean County, Korea; January 4 to February 19 2008 | a CS Survey; Clean-up workers—Military personnel; n = 2624 | Hebei Spirit oil | Structured self-assessment Questionnaire | Cough, sore throat, runny nose, dry mouth, sputum, with younger age group having fewer symptoms than the older age groups. | - |
| 16. | [53]; Taean Area, Korea; December 13 to 20, 2007 | a CS Survey; Residents, Volunteers and Clean-up workers; (n = 846) | Hebei Spirit oil | Questionnaire; Interviews | Respiratory symptoms (sore throat, cough, respiratory discomforts) OR: 1.5 (1.3–1.8). | Age, female/male, status, duration of clean-up activities, and hours worked per day |
| 17. | [54]; Atlantic Coast/Cantabrian Coast, Spain; September 2004 and February 2005 | a CS; Clean-up workers (Fishermen and women) exposed = 501; non-exposed= 177 | Prestige oil | Questionnaire survey, Interviews; Spirometry | Risks for symptoms of lower respiratory tract diseases elevated (RD: 8.0 [95% CI: 1.0 to 15.0]), increased biomarkers of airway injury in the exposed. No remarkable difference in pulmonary function between the two categories. | Sex, smoking status |
| 18. | [56]; Asturia and Cantabria, Spain; 29 November 2002 to 21 July 2003 | Census survey; Clean-up workers- Sea men, volunteers, bird cleaners, paid workers (n = 799) | Prestige oil | Structured questionnaire; computer-assisted telephonic interviews | A statistically significant association between Prestige Spirit oil spill exposure and throat and respiratory health issues OR: 10.4 [95% CI 4.0–27.4] p < 0.001 *. | - |
| 19. | [55]; Asturia and Cantabria, Spain; 2008 | A follow-up study; Clean-up workers; n = 501 exposed vs. n = 177 unexposed | Prestige oil | Structured validated questionnaire; computer-assisted telephonic interviews | Higher prevalence of lower respiratory tract symptoms (e.g., wheeze, dyspnoea, cough, and sputum production) in the exposed (RR 1.4, 95% CI 1.0–2.0). With increase in the degree of exposure, there was a corresponding increased risk of chronic respiratory symptoms: RR: 1.7 (95% CI 0.9–3.1) and 3.3 (95% CI 1.8–6.0) for averagely and profoundly exposed, respectively, as against those that were symptomless. | Sex, age, smoking status |
| 20. | [57]; Spain; November 2008–April 2009 | A follow-up longitudinal study; Clean-up workers (Exposed vs. Unexposed Fishermen); (n = 160 vs. 60) | Prestige oil | Respiratory function testing (spirometry); Methacholine challenge test | Similar or better respiratory health statistics were observed in oil spill responders when compared to the non-exposed 6 years after the spill (FEV1/FVC and FEF25–75% were increased remarkably). | Smokers |
| Selection | Comparability | Outcome | Remark | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S/N | Reference | Representativeness | Selection of Comparison Group | Ascertainment of Exposure | Precision of Exposure Dose Ascertainment | Ascertainment of Exposure Performed Prospectively or Retrospectively | Demonstration That Outcome of Interest Was Not Present at Start of Study, OR Baseline Assessment | Adjustment for Confounding (Rendering Comparability of Cohorts on the Basis of the Design or Analysis) | Assessment of Outcome | Was Follow-Up Long Enough for Outcomes to Occur? | Adequacy of Follow-Up of Cohorts | Remark Risk of Bias (High, Medium, Low) |
| 1. | [39] | 0 | + | + | 0 | 0 | + | ++ | + | + | + | 9 |
| 2. | [40] | 0 | + | + | 0 | 0 | 0 | ++ | + | 0 | 0 | 5 |
| 3. | [41] | 0 | + | + | 0 | 0 | 0 | ++ | + | 0 | 0 | 5 |
| 4. | [49] | + | + | 0 | 0 | + | + | 0 | + | + | + | 7 |
| 5. | [51] | + | + | 0 | 0 | + | + | + | + | + | + | 8 |
| 6. | [50] | + | + | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| 7. | [42] | 0 | + | 0 | 0 | + | 0 | 0 | 0 | + | 0 | 3 |
| 8. | [8] | + | + | + | + | + | 0 | + | 0 | 0 | 0 | 6 |
| 9. | [43] | + | + | 0 | 0 | + | 0 | ++ | + | 0 | 0 | 6 |
| 10. | [44] | + | + | + | 0 | + | 0 | ++ | + | 0 | 0 | 7 |
| 11. | [45] | + | + | + | + | + | 0 | ++ | + | 0 | 0 | 8 |
| 12. | [48] | + | + | + | + | + | + | ++ | + | 0 | 0 | 9 |
| 13. | [46] | + | + | 0 | + | + | 0 | ++ | + | + | 0 | 8 |
| 14. | [47] | + | + | 0 | 0 | + | 0 | ++ | + | 0 | 0 | 6 |
| 15. | [52] | 0 | 0 | + | 0 | 0 | 0 | 0 | + | 0 | 0 | 2 |
| 16. | [53] | 0 | 0 | + | 0 | 0 | 0 | ++ | 0 | 0 | 0 | 3 |
| 17. | [54] | + | + | 0 | 0 | 0 | 0 | ++ | + | 0 | 0 | 5 |
| 18. | [56] | + | 0 | 0 | 0 | 0 | 0 | 0 | + | + | 0 | 3 |
| 19. | [55] | + | + | + | 0 | + | 0 | ++ | 0 | + | + | 8 |
| 20. | [57] | + | + | 0 | 0 | + | 0 | + | + | + | + | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abereton, P.; Ordinioha, B.; Mensah-Attipoe, J.; Toyinbo, O. Crude Oil Spills and Respiratory Health of Clean-Up Workers: A Systematic Review of Literature. Atmosphere 2023, 14, 494. https://doi.org/10.3390/atmos14030494
Abereton P, Ordinioha B, Mensah-Attipoe J, Toyinbo O. Crude Oil Spills and Respiratory Health of Clean-Up Workers: A Systematic Review of Literature. Atmosphere. 2023; 14(3):494. https://doi.org/10.3390/atmos14030494
Chicago/Turabian StyleAbereton, Pearl, Best Ordinioha, Jacob Mensah-Attipoe, and Oluyemi Toyinbo. 2023. "Crude Oil Spills and Respiratory Health of Clean-Up Workers: A Systematic Review of Literature" Atmosphere 14, no. 3: 494. https://doi.org/10.3390/atmos14030494