Sex Differences in Colon Cancer: Genomic and Nongenomic Signalling of Oestrogen
Abstract
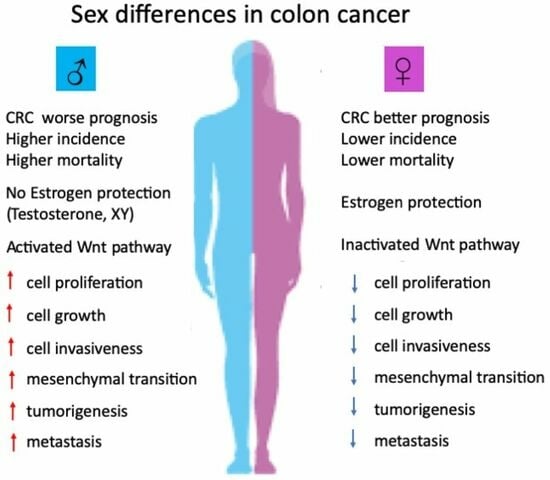
:1. Introduction
1.1. Sex Differences/Sexual Dimorphism/Gender Differences in Cancer
1.2. Sex Differences and Regional Variances in Colon Cancer
1.3. Oestrogen and Sex Differences in Colon Cancer
2. Oestrogen Receptors in Colon Cancer
2.1. Nuclear Oestrogen Receptors in Colon Cancer
2.2. Membrane Oestrogen Receptors in Colon Cancer
3. Genomic and Nongenomic Oestrogen Signalling Pathways in Colon Cancer
3.1. Genomic Mechanisms of Oestrogen Signalling in Colon Cancer
3.2. Nongenomic Mechanisms of Oestrogen Signalling in Colon Cancer
3.3. Cooperativity between Genomic and Nongenomic Oestrogen Signalling in Colon Cancer
4. Oestrogen Signalling via ERα and ERβ in Colon Cancer
4.1. Nonligand Activation of Nuclear ERs in Colon Cancer
4.2. Membrane Oestrogen Receptors mERα and mERβ in Colon Cancer
4.3. Oestrogen Signalling via Truncated ERs in Colon Cancer
5. Oestrogen Signalling via G Protein-Coupled Oestrogen Receptor in Colon Cancer
Hypoxia and GPER Signalling in Colon Cancer
6. Oestrogen Regulation of Wnt/β-Catenin Signalling in Colon Cancer
6.1. Oestrogen Regulation of Wnt-KCNQ1 Interactions in Colon Cancer
6.2. Oestrogen Regulation of Wnt Receptor Oncogenic Signalling in Colon Cancer
7. Oestrogen Regulation of Epigenetic, Microbiome and Metabolic Factors in Colon Cancer
8. Comparison of Oestrogen and Testosterone Signalling in Colon Cancer
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubin, J.B.; Lagas, J.S.; Broestl, L.; Sponagel, J.; Rockwell, N.; Rhee, G.; Rosen, S.F.; Chen, S.; Klein, R.S.; Imoukhuede, P.; et al. Sex differences in cancer mechanisms. Biol. Sex Differ. 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Dorak, M.T. Sexual dimorphism in molecular biology of cancer. In Principles of Gender-Specific Medicine, 4th ed.; Legato, M.J., Ed.; Academic Press: Cambridge, MA, USA, 2023; Chapter 29; pp. 463–476. [Google Scholar] [CrossRef]
- Lassek, W.D.; Gaulin, S.J.C. Substantial but Misunderstood Human Sexual Dimorphism Results Mainly From Sexual Selection on Males and Natural Selection on Females. Front. Psychol. 2022, 13, 859931. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V. Sex and gender differences in health. Science & Society Series on Sex and Science. EMBO Rep. 2012, 13, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Maney, D.L. Perils and pitfalls of reporting sex differences. Phil. Trans. R. Soc. 2016, 371, 20150119. [Google Scholar] [CrossRef]
- Zheng, D.; Trynda, J.; Williams, C.; Vold, J.A.; Nguyen, J.H.; Harnois, D.M.; Bagaria, S.P.; McLaughlin, S.A.; Li, Z. Sexual dimorphism in the incidence of human cancers. BMC Cancer 2019, 19, 684. [Google Scholar] [CrossRef]
- Kim, H.I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Rubin, J.B. The spectrum of sex differences in cancer. Trends Cancer 2022, 8, 303–315. [Google Scholar] [CrossRef]
- Jacob, L.; Freyn, M.; Kalder, M.; Dinas, K.; Kostev, K. Impact of tobacco smoking on the risk of developing 25 different cancers in the UK: A retrospective study of 422,010 patients followed for up to 30 years. Oncotarget 2018, 9, 17420–17429. [Google Scholar] [CrossRef]
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080. [Google Scholar] [CrossRef]
- Abancens, M.; Bustos, V.; Harvey, H.; McBryan, J.; Harvey, B.J. Sexual Dimorphism in Colon Cancer. Front. Oncol. 2020, 10, 607909. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, G.; He, J.; Ren, S.; Wu, F.; Zhang, J.; Wang, F. Gender Differences in Colorectal Cancer Survival: A Meta-Analysis. Int. J. Cancer 2017, 141, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Globocan. Estimated Cancer Incidence, Mortality and Prevalence Worldwide 2018. 2018. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 29 July 2023).
- GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022, 7, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory. Available online: https://gco.iarc.fr/tomorrow/en/dataviz/trends?types=0_1&sexes=1_2&mode=population&group_populations=0&multiple_populations=1&multiple_cancers=1&cancers=8&populations=994&apc=cat_ca20v1.5_ca23v-1.5&group_cancers=1 (accessed on 29 July 2023).
- Hogan, A.M.; Collins, D.; Baird, A.W.; Winter, D.C. Oestrogen and gastrointestinal malignancy. Mol. Cell. Endocrinol. 2009, 307, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.H.; Jalaludin, B.; Wong, S.K.; Kneebone, A.; Connor, S.J.; Leong, R.W. Improved survival in young women with colorectal cancer. Am. J. Gastroenterol. 2008, 103, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Rennert, G.; Rennert, H.S.; Pinchev, M.; Lavie, O.; Gruber, S.B. Use of hormone replacement therapy and the risk of colorectal cancer. J. Clin. Oncol. 2009, 27, 4542–4547. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Humphrey, L.L.; Nygren, P.; Teutsch, S.M.; Allan, J.D. Postmenopausal hormone replacement therapy: Scientific review. JAMA 2002, 288, 872–881. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Wactawski-Wende, J.; Ritenbaugh, C.; Hubbell, F.A.; Ascensao, J.; Rodabough, R.J.; Rosenberg, C.A.; Taylor, V.M.; Harris, R.; Chen, C.; et al. Oestrogen plus progestin and colorectal cancer in postmenopausal women. N. Engl. J. Med. 2004, 350, 991–1004. [Google Scholar] [CrossRef]
- Kennelly, R.; Kavanagh, D.O.; Hogan, A.M.; Winter, D.C. Oestrogen and the colon: Potential mechanisms for cancer prevention. Lancet Oncol. 2008, 9, 385–391. [Google Scholar] [CrossRef]
- Caiazza, F.; Ryan, E.J.; Doherty, G.; Winter, D.C.; Sheahan, K. Oestrogen receptors and their implications in colorectal carcinogenesis. Front. Oncol. 2015, 5, 19. [Google Scholar] [CrossRef]
- Perotti, V.; Fabiano, S.; Contiero, P.; Michiara, M.; Musolino, A.; Boschetti, L.; Cascone, G.; Castelli, M.; Tagliabue, G.; Cancer Registries Working Group. Influence of Sex and Age on Site of Onset, Morphology, and Site of Metastasis in Colorectal Cancer: A Population-Based Study on Data from Four Italian Cancer Registries. Cancers 2023, 15, 803. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Menter, D.G.; Kopetz, S. Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes. J. Natl. Compr. Cancer Netw. 2017, 15, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Mik, M.; Berut, M.; Dziki, L.; Trzcinski, R.; Dziki, A. Right- and left-sided colon cancer—Clinical and pathological differences of the disease entity in one organ. Arch. Med. Sci. 2017, 13, 157–162. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Ironmonger, L.; Steele, R.J.C.; Ormiston-Smith, N.; Crawford, C.; Seims, A. A review of sex-related differences in colorectal cancer incidence, screening uptake, routes to diagnosis, cancer stage and survival in the UK. BMC Cancer 2018, 18, 906. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Richman, S.; Adlard, J. Left and right sided large bowel cancer: Have significant genetic differences in addition to well-known clinical differences. BMJ Br. Med. J. 2002, 324, 931–932. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.S.; van Neerven, S.M.; Pleguezuelos-Manzano, C.; Simmini, S.; Léveillé, N.; de Groot, N.E.; Holding, A.N.; Markowetz, F.; Vermeulen, L. Intestinal region-specific Wnt signalling profiles reveal interrelation between cell identity and oncogenic pathway activity in cancer development. Cancer Cell Int. 2020, 20, 578. [Google Scholar] [CrossRef] [PubMed]
- Leedham, S.J.; Rodenas-Cuadrado, P.; Howarth, K.; Lewis, A.; Mallappa, S.; Segditsas, S.; Davis, H.; Jeffery, R.; Rodriguez-Justo, M.; Keshav, S.; et al. A basal gradient of Wnt and stem-cell number influences regional tumour distribution in human and mouse intestinal tracts. Gut 2013, 62, 83–93. [Google Scholar] [CrossRef]
- Lopes-Ramos, C.M.; Kuijjer, M.L.; Ogino, S.; Fuchs, C.S.; DeMeo, D.L.; Glass, K.; Quackenbush, J. Gene Regulatory Network Analysis Identifies Sex-Linked Differences in Colon Cancer Drug Metabolism. Cancer Res. 2018, 78, 5538–5547. [Google Scholar] [CrossRef]
- Disoma, C.; Zhou, Y.; Li, S.; Peng, J.; Xia, Z. Wnt/β-catenin signalling in colorectal cancer: Is therapeutic targeting even possible? Biochimie 2022, 195, 39–53. [Google Scholar] [CrossRef]
- Dupon, C.; Hosseinian, A.; Kim, M.H. Simultaneous determination of plasma estrogens, androgens, and progesterone during the human menstrual cycle. Steroids 1973, 22, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Shutt, D.A.; Smith, I.D.; Shearman, R.P. Oestrone, oestradiol-17beta and oestriol levels in human foetal plasma during gestation and at term. J. Endocrinol. 1974, 60, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Banerjee, A.; Singh, D.; Thakur, G.; Kasarpalkar, N.; Gavali, S.; Gadkar, S.; Madan, T.; Mahale, S.D.; Balasinor, N.H.; et al. Estradiol: A Steroid with Multiple Facets. Horm. Metab. Res. 2018, 50, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.C.; Garratt, M.G. Life history evolution, reproduction, and the origins of sex-dependent aging and longevity. Ann. N. Y. Acad. Sci. 2017, 1389, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, K.J.; Hewitt, S.C.; Arao, Y.; Korach, K.S. Oestrogen Hormone Biology. Curr. Top. Dev. Biol. 2017, 125, 109–146. [Google Scholar] [CrossRef] [PubMed]
- Barzi, A.; Lenz, A.M.; Labonte, M.J.; Lenz, H.J. Molecular pathways: Oestrogen pathway in colorectal cancer. Clin. Cancer Res. 2013, 19, 5842–5848. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, M.; Haseefa, F.; Peyton, L.; Park, S.; Movahed, M.R. The effects of oestrogen and hormone replacement therapy on platelet activity: A review. Am. J. Blood Res. 2022, 12, 33–42. [Google Scholar] [PubMed]
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef]
- Das, P.K.; Saha, J.; Pillai, S.; Lam, A.K.; Gopalan, V.; Islam, F. Implications of oestrogen and its receptors in colorectal carcinoma. Cancer Med. 2023, 12, 4367–4379. [Google Scholar] [CrossRef]
- Moggs, J.G.; Orphanides, G. Oestrogen receptors: Orchestrators of pleiotropic cellular responses. EMBO Rep. 2001, 2, 775–781. [Google Scholar] [CrossRef]
- Topi, G.; Ghatak, S.; Satapathy, S.R.; Ehrnström, R.; Lydrup, M.L.; Sjölander, A. Combined Oestrogen Alpha and Beta Receptor Expression Has a Prognostic Significance for Colorectal Cancer Patients. Front. Med. 2022, 9, 739620. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.L.; Xu, X.; Norfleet, A.M.; Watson, C.S. The presence of functional oestrogen receptors in intestinal epithelial cells. Endocrinology 1993, 132, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.L.; Javid, S.H.; Carothers, A.M.; Redston, M.; Bertagnolli, M.M. Oestrogen receptors α and β are inhibitory modifiers of Apc-dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res. 2007, 67, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; DiLeo, A.; Niv, Y.; Gustafsson, J.Å. Oestrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016, 372, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Foley, E.F.; Jazaeri, A.A.; Shupnik, M.A.; Jazaeri, O.; Rice, L.W. Selective loss of oestrogen receptor β in malignant human colon. Cancer Res. 2000, 60, 245–248. [Google Scholar] [PubMed]
- Baraibar, I.; Ros, J.; Saoudi, N.; Salvà, F.; García, A.; Castells, M.; Tabernero, J.; Élez, E. Sex and gender perspectives in colorectal cancer. ESMO Open 2023, 8, 101204. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Gustafsson, J.A. Oestrogen Signalling: A Subtle Balance Between ER alpha and ER beta. Mol. Interv. 2003, 3, 281–292. [Google Scholar] [CrossRef]
- Ditonno, I.; Losurdo, G.; Rendina, M.; Pricci, M.; Girardi, B.; Ierardi, E.; Di Leo, A. Oestrogen Receptors in Colorectal Cancer: Facts, Novelties and Perspectives. Curr. Oncol. 2021, 28, 4256–4263. [Google Scholar] [CrossRef]
- Niv, Y. Estrogen receptor β expression and colorectal cancer: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1438–1442. [Google Scholar] [CrossRef]
- Nikolaou, S.; Qiu, S.; Fiorentino, F.; Rasheed, S.; Tekkis, P.; Kontovounisios, C. The prognostic and therapeutic role of hormones in colorectal cancer: A review. Mol. Biol. Rep. 2019, 46, 1477–1486. [Google Scholar] [CrossRef]
- Maingi, J.W.; Tang, S.; Liu, S.; Ngenya, W.; Bao, E. Targeting oestrogen receptors in colorectal cancer. Mol. Biol. Rep. 2020, 47, 4087–4091. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Razandi, M.; Levin, E.R. Nature of functional oestrogen receptors at the plasma membrane. Mol. Endocrinol. 2006, 20, 1996–2009. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, F.; Thomas, W.; Harvey, B.J. Novel female sex-dependent actions of oestrogen in the intestine. J. Physiol. 2009, 587 Pt 21, 5039–5044. [Google Scholar] [CrossRef] [PubMed]
- Jacquot, Y.; de Cremoux, P.; Harvey, B.J.; Wehling, M. The Rapid Responses to Steroid Hormones meetings: An important event for steroid science. Ann. Endocrinol. 2023, 84, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Barton, M.; Frigo, D.E.; Madak-Erdogan, Z.; Mauvais-Jarvis, F.; Prossnitz, E.R. Steroid Hormones and Receptors in Health and Disease: A Research Conference Co-Organized by FASEB and the International Committee on Rapid Responses to Steroid Hormones [RRSH], May 25–27, 2021. FASEB J. 2021, 35, e21858. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.J. Guest editorial: 11th international meeting on rapid responses to steroid hormones RRSH2018. Steroids 2020, 154, 108552. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Plasma membrane oestrogen receptors. Trends Endocrinol. Metab. 2009, 20, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Beswick, E.J.; Krajewska, W.M.; Prossnitz, E.R. G Protein-Coupled Oestrogen Receptor in Colon Function, Immune Regulation and Carcinogenesis. World J. Gastroenterol. 2019, 25, 4092–4104. [Google Scholar] [CrossRef]
- Wilkenfeld, S.R.; Lin, C.; Frigo, D.E. Communication between genomic and non-genomic signalling events coordinate steroid hormone actions. Steroids 2018, 133, 2–7. [Google Scholar] [CrossRef]
- Levin, E.R.; Hammes, S.R. Nuclear receptors outside the nucleus: Extranuclear signalling by steroid receptors. Nat. Rev. Mol. Cell Biol. 2016, 17, 783–797. [Google Scholar] [CrossRef]
- O’Mahony, F.; Harvey, B.J. Sex and oestrous cycle-dependent rapid protein kinase signalling actions of oestrogen in distal colonic cells. Steroids 2008, 73, 889–894. [Google Scholar] [CrossRef]
- Fuentes, N.; Silveyra, P. Oestrogen receptor signalling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Levin, E.R. Extranuclear oestrogen receptor’s roles in physiology: Lessons from mouse models. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E133–E140. [Google Scholar] [CrossRef]
- Konstantinopoulos, P.A.; Kominea, A.; Vandoros, G.; Sykiotis, G.P.; Andricopoulos, P.; Varakis, I.; Sotiropoulou-Bonikou, G.; Papavassiliou, A.G. Oestrogen receptor beta (ERbeta) is abundantly expressed in normal colonic mucosa, but declines in colon adenocarcinoma paralleling the tumour’s dedifferentiation. Eur. J. Cancer 2003, 39, 1251–1258. [Google Scholar] [CrossRef]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Forootan, F.S.; Esfahani, M.H.N.; Ghaedi, K. Signalling pathways involved in colorectal cancer progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef]
- Losordo, D.W.; Isner, J.M. Oestrogen and angiogenesis: A review. Arter. Thromb. Vasc. Biol. 2001, 21, 6–12. [Google Scholar] [CrossRef]
- Gargett, C.E.; Zaitseva, M.; Bucak, K.; Chu, S.; Fuller, P.J.; Rogers, P.A. 17-beta-estradiol up-regulates vascular endothelial growth factor receptor-2 expression in human myometrial microvascular endothelial cells: Role of oestrogen receptor-alpha and -beta. J. Clin. Endocrinol. Metab. 2002, 87, 4341–4349. [Google Scholar] [CrossRef]
- Grady, W.M.; Yu, M.; Markowitz, S.D. Epigenetic Alterations in the Gastrointestinal Tract: Current and Emerging Use for Biomarkers of Cancer. Gastroenterology 2021, 160, 690–709. [Google Scholar] [CrossRef]
- Cignarella, A.; Boscaro, C.; Albiero, M.; Bolego, C.; Barton, M. Post-transcriptional and epigenetic regulation of oestrogen signalling. J. Pharmacol. Exp. Ther. 2023, 386, 288–297. [Google Scholar] [CrossRef]
- Yoriki, K.; Mori, T.; Kokabu, T.; Matsushima, H.; Umemura, S.; Tarumi, Y.; Kitawaki, J. Oestrogen-related receptor alpha induces epithelial-mesenchymal transition through cancer-stromal interactions in endometrial cancer. Sci. Rep. 2019, 9, 6697. [Google Scholar] [CrossRef]
- Vu, T.; Datta, P.K. Regulation of EMT in Colorectal Cancer: A Culprit in Metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Bilancio, A.; Perillo, B.; Sinisi, A.A.; Migliaccio, A.; Castoria, G. Oestrogen Receptors in Epithelial-Mesenchymal Transition of Prostate Cancer. Cancers 2019, 11, 1418. [Google Scholar] [CrossRef] [PubMed]
- Bouris, P.; Skandalis, S.S.; Piperigkou, Z.; Afratis, N.; Karamanou, K.; Aletras, A.J.; Moustakas, A.; Theocharis, A.D.; Karamanos, N.K. Oestrogen receptor alpha mediates epithelial to mesenchymal transition, expression of specific matrix effectors and functional properties of breast cancer cells. Matrix Biol. 2015, 43, 42–60. [Google Scholar] [CrossRef] [PubMed]
- Orzołek, I.; Sobieraj, J.; Domagała-Kulawik, J. Estrogens, Cancer and Immunity. Cancers 2022, 14, 2265. [Google Scholar] [CrossRef] [PubMed]
- Zafari, N.; Khosravi, F.; Rezaee, Z.; Esfandyari, S.; Bahiraei, M.; Bahramy, A.; Ferns, G.A.; Avan, A. The role of the tumour microenvironment in colorectal cancer and the potential therapeutic approaches. J. Clin. Lab. Anal. 2022, 36, e24585. [Google Scholar] [CrossRef] [PubMed]
- Hases, L.; Archer, A.; Williams, C. ERβ and Inflammation. Adv. Exp. Med. Biol. 2022, 1390, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Doolan, C.M.; Condliffe, S.B.; Harvey, B.J. Rapid non-genomic activation of cytosolic cyclic AMP-dependent protein kinase activity and [Ca2+]i by 17beta-oestradiol in female rat distal colon. Br. J. Pharmacol. 2000, 129, 1375–1386. [Google Scholar] [CrossRef] [PubMed]
- Arterburn, J.B.; Prossnitz, E.R. G Protein-Coupled Oestrogen Receptor GPER: Molecular Pharmacology and Therapeutic Applications. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 295–320. [Google Scholar] [CrossRef]
- Simoncini, T.; Genazzani, A.R. Non-genomic actions of sex steroid hormones. Eur. J. Endocrinol. 2003, 148, 281–292. [Google Scholar] [CrossRef]
- Slattery, M.L.; Lundgreen, A.; Wolff, R.K. MAP kinase genes and colon and rectal cancer. Carcinogenesis 2012, 33, 2398–2408. [Google Scholar] [CrossRef]
- Irnaten, M.; Blanchard-Gutton, N.; Harvey, B.J. Rapid effects of 17beta-estradiol on epithelial TRPV6 Ca2+ channel in human T84 colonic cells. Cell Calcium 2008, 44, 441–452. [Google Scholar] [CrossRef]
- O’Mahony, F.; Alzamora, R.; Betts, V.; LaPaix, F.; Carter, D.; Irnaten, M.; Harvey, B.J. Female gender-specific inhibition of KCNQ1 channels and chloride secretion by 17beta-estradiol in rat distal colonic crypts. J. Biol. Chem. 2007, 282, 24563–24573. [Google Scholar] [CrossRef] [PubMed]
- Prevarskaya, N.; Skryma, R.; Shuba, Y. Ion Channels in Cancer: Are Cancer Hallmarks Oncochannelopathies? Physiol. Rev. 2018, 98, 559–621. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Pallottini, V.; Trentalance, A. Estrogens cause rapid activation of IP3-PKC-alpha signal transduction pathway in HEPG2 cells. Biochem. Biophys. Res. Commun. 1998, 245, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Doolan, C.M.; Harvey, B.J. Modulation of cytosolic protein kinase C and calcium ion activity by steroid hormones in rat distal colon. J. Biol. Chem. 1996, 271, 8763–8767. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Marino, M. Synergism between genomic and non genomic oestrogen action mechanisms. IUBMB Life 2003, 55, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pedram, A.; Razandi, M.; Aitkenhead, M.; Hughes, C.C.; Levin, E.R. Integration of the non-genomic and genomic actions of oestrogen. Membrane-initiated signalling by steroid to transcription and cell biology. J. Biol. Chem. 2002, 277, 50768–50775. [Google Scholar] [CrossRef] [PubMed]
- Björnström, L.; Sjöberg, M. Mechanisms of Oestrogen Receptor Signalling: Convergence of Genomic and Nongenomic Actions on Target Genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef]
- Martin, M.B.; Franke, T.F.; Stoica, G.E.; Chambon, P.; Katzenellenbogen, B.S.; Stoica, B.A.; McLemore, M.S.; Olivo, S.E.; Stoica, A. A role for Akt in mediating the estrogenic functions of epidermal growth factor and insulin-like growth factor I. Endocrinology 2000, 141, 4503–4511. [Google Scholar] [CrossRef]
- Kato, S.; Endoh, H.; Masuhiro, Y.; Kitamoto, T.; Uchiyama, S.; Sasaki, H.; Masushige, S.; Gotoh, Y.; Nishida, E.; Kawashima, H.; et al. Activation of the oestrogen receptor through phosphorylation by mitogen-activated protein kinase. Science 1995, 270, 1491–1494. [Google Scholar] [CrossRef]
- Levin, E.R. Integration of the Extranuclear and Nuclear Actions of Oestrogen. Mol. Endocrinol. 2005, 19, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Madak-Erdogan, Z.; Lupien, N.; Stossi, F.; Brown, M.; Katzenellenbogen, B.S. Genomic Collaboration of Oestrogen Receptor α and Extracellular Signal-Regulated Kinase 2 in Regulating Gene and Proliferation Programs. Mol. Cell. Biol. 2011, 31, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, B.A.; Harvey, B.J.; Healy, V. 17beta-Estradiol rapidly stimulates c-fos expression via the MAPK pathway in T84 cells. Mol. Cell. Endocrinol. 2005, 229, 39–47. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, F.; Alzamora, R.; Chung, H.L.; Thomas, W.; Harvey, B.J. Genomic priming of the antisecretory response to oestrogen in rat distal colon throughout the oestrous cycle. Mol. Endocrinol. 2009, 23, 1885–1899. [Google Scholar] [CrossRef] [PubMed]
- Wade, C.B.; Robinson, S.; Shapiro, R.A.; Dorsa, D.M. Oestrogen receptor [ER]alpha and ERbeta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology 2001, 142, 2336–2342. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Membrane oestrogen receptors signal to determine transcription factor function. Steroids 2018, 132, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cato, A.C.; Nestl, A.; Mink, S. Rapid actions of steroid receptors in cellular signaling pathways. Sci. STKE 2002, 2002, re9. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Galluzzo, P.; Ascenzi, P. Oestrogen signalling multiple pathways to impact gene transcription. Curr Genom. 2006, 7, 497–508. [Google Scholar] [CrossRef]
- Khatpe, A.S.; Adebayo, A.K.; Herodotou, C.A.; Kumar, B.; Nakshatri, H. Nexus between PI3K/AKT and Oestrogen Receptor Signalling in Breast Cancer. Cancers 2021, 13, 369. [Google Scholar] [CrossRef]
- Mal, R.; Magner, A.; David, J.; Datta, J.; Vallabhaneni, M.; Kassem, M.; Manouchehri, J.; Willingham, N.; Stover, D.; Vandeusen, J.; et al. Oestrogen Receptor beta [ERβ]: A Ligand Activated Tumour Suppressor. Front Oncol. 2020, 10, 587386. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L.; Hwang, P.M.; Kinzler, K.W.; Vogelstein, B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol. Cell 2001, 7, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Barros, R.P.; Warner, M.; Ström, A.; Gustafsson, J.Å. Insight into the mechanisms of action of oestrogen receptor β in the breast, prostate, colon, and CNS. J. Mol. Endocrinol. 2013, 51, T61–T74. [Google Scholar] [CrossRef] [PubMed]
- Topi, G.; Satapathy, S.R.; Dash, P.; Fred Mehrabi, S.; Ehrnström, R.; Olsson, R.; Lydrup, M.L.; Sjölander, A. Tumour-suppressive effect of oestrogen receptor β in colorectal cancer patients, colon cancer cells, and a zebrafish model. J. Pathol. 2020, 251, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Waters, C.E.; Lewis, A.E.; Langman, M.J.; Eggo, M.C. Oestrogen-induced apoptosis in colonocytes expressing oestrogen receptor beta. J. Endocrinol. 2002, 174, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, K.; Ström, A.; Jonsson, P.; Gustafsson, J.Å.; Williams, C. Estrogen receptor β induces antiinflammatory and antitumorigenic networks in colon cancer cells. Mol. Endocrinol. 2011, 25, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Martineti, V.; Picariello, L.; Tognarini, I.; Sala, S.C.; Gozzini, A.; Azzari, C.; Mavilia, C.; Tanini, A.; Falchetti, A.; Fiorelli, G.; et al. ERbeta is a potent inhibitor of cell proliferation in the HCT8 human colon cancer cell line through regulation of cell cycle components. Endocr. Relat. Cancer 2005, 12, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Huang, C.; Wu, H.; Huang, J. Oestrogen Receptor beta [ERβ] Mediated-CyclinD1 Degradation via Autophagy Plays an Anti-Proliferation Role in Colon Cells. Int. J. Biol. Sci. 2019, 15, 942–952. [Google Scholar] [CrossRef]
- Hases, L.; Indukuri, R.; Birgersson, M.; Nguyen-Vu, T.; Lozano, R.; Saxena, A.; Hartman, J.; Frasor, J.; Gustafsson, J.; Katajisto, P.; et al. Intestinal oestrogen receptor beta suppresses colon inflammation and tumorigenesis in both sexes. Cancer Lett. 2020, 492, 54–62. [Google Scholar] [CrossRef]
- Braniste, V.; Leveque, M.; Buisson-Brenac, C.; Bueno, L.; Fioramonti, J.; Houdeau, E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J. Physiol. 2009, 587 Pt 13, 3317–3328. [Google Scholar] [CrossRef]
- Silva, R.S.; Lombardi, A.P.G.; de Souza, D.S.; Vicente, C.M.; Porto, C.S. Activation of oestrogen receptor beta [ERβ] regulates the expression of N-cadherin, E-cadherin and β-catenin in androgen-independent prostate cancer cells. Int. J. Biochem. Cell Biol. 2018, 96, 40–50. [Google Scholar] [CrossRef]
- Nguyen-Vu, T.; Wang, J.; Mesmar, F.; Mukhopadhyay, S.; Saxena, A.; McCollum, C.W.; Gustafsson, J.Å.; Bondesson, M.; Williams, C. Oestrogen receptor beta reduces colon cancer metastasis through a novel miR-205—PROX1 mechanism. Oncotarget 2016, 7, 42159–42171. [Google Scholar] [CrossRef]
- Hases, L.; Archer, A.; Indukuri, R.; Birgersson, M.; Savva, C.; Korach-André, M.; Williams, C. High-fat diet and oestrogen impacts the colon and its transcriptome in a sex-dependent manner. Sci. Rep. 2020, 10, 16160. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.; Wihlén, B.; Tujague, M.; Wan, J.; Ström, A.; Gustafsson, J.A. Oestrogen receptor [ER] beta modulates ERalpha-mediated transcriptional activation by altering the recruitment of c-Fos and c-Jun to oestrogen-responsive promoters. Mol. Endocrinol. 2006, 20, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Gougelet, A.; Mueller, S.O.; Korach, K.S.; Renoir, J.M. Oestrogen receptors pathways to oestrogen responsive elements: The transactivation function-1 acts as the keystone of oestrogen receptor [ER]beta-mediated transcriptional repression of ERalpha. J. Steroid Biochem. Mol. Biol. 2007, 104, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Paech, K.; Webb, P.; Kuiper, G.G.; Nilsson, S.; Gustafsson, J.Å.; Kushner, P.J.; Scanlan, T.S. Differential ligand activation of oestrogen receptors ERalpha and ERbeta at AP1 sites. Science 1997, 277, 1508–1510. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.G.; Oikarinen, S.I.; Bynoté, K.K.; Marttinen, M.; Rafter, J.J.; Gustafsson, J.; Roy, S.K.; Pitot, H.C.; Korach, K.S.; Lubahn, D.B.; et al. Disruption of oestrogen receptor signalling enhances intestinal neoplasia in Apc[Min/+] mice. Carcinogenesis 2009, 30, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Nüssler, N.C.; Reinbacher, K.; Shanny, N.; Schirmeier, A.; Glanemann, M.; Neuhaus, P.; Nussler, A.K.; Kirschner, M. Sex-specific differences in the expression levels of estrogen receptor subtypes in colorectal cancer. Gend Med. 2008, 5, 209–217. [Google Scholar] [CrossRef]
- Xie, L.Q.; Yu, J.P.; Luo, H.S. Expression of oestrogen receptor beta in human colorectal cancer. World J. Gastroenterol. 2004, 10, 214–217. [Google Scholar] [CrossRef]
- 122. Grivas, P.D.; Tzelepi, V.; Sotiropoulou-Bonikou, G.; Kefalopoulou, Z.; Papavassiliou, A.G.; Kalofonos, H. Expression of ERalpha, ERbeta and co-regulator PELP1/MNAR in colorectal cancer: Prognostic significance and clinicopathologic correlations. Cell Oncol. 2009, 31, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Stevanato Filho, P.R.; Aguiar Junior, S.; Begnami, M.D.; Ferreira, F.D.O.; Nakagawa, W.T.; Spencer, R.M.S.B.; Bezerra, T.S.; Boggiss, P.E.; Lopes, A. Oestrogen receptor β as a prognostic marker of tumour progression in colorectal cancer with familial adenomatous polyposis and sporadic polyps. Pathol. Oncol. Res. 2018, 24, 533–540. [Google Scholar] [CrossRef]
- Herichova, I.; Reis, R.; Hasakova, K.; Vician, M.; Zeman, M. Sex-dependent regulation of oestrogen receptor beta in human colorectal cancer tissue and its relationship with clock genes and VEGF-A expression. Physiol. Res. 2019, 68 (Suppl. 3), S297–S305. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R. Levin, Bidirectional Signalling between the Oestrogen Receptor and the Epidermal Growth Factor Receptor. Mol. Endocrinol. 2003, 17, 309–317. [Google Scholar] [CrossRef]
- Ahcene Djaballah, S.; Daniel, F.; Milani, A.; Ricagno, G.; Lonardi, S. HER2 in Colorectal Cancer: The Long and Winding Road From Negative Predictive Factor to Positive Actionable Target. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Krasinskas, A.M. EGFR Signalling in Colorectal Carcinoma. Pathol. Res. Int. 2011, 2011, 932932. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.-F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Chambon, P.; Katzenellenbogen, B.; et al. Membrane and Nuclear Oestrogen Receptor Alpha Actions: From Tissue Specificity to Medical Implications. Physiol. Rev. 2017, 97, 1045–1087. [Google Scholar] [CrossRef] [PubMed]
- Norfleet, A.M.; Thomas, M.L.; Gametchu, B.; Watson, C.S. Oestrogen receptor-alpha detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumour cells by enzyme-linked immunocytochemistry. Endocrinology 1999, 140, 3805–3814. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Extranuclear steroid receptors are essential for steroid hormone actions. Annu. Rev. Med. 2015, 66, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Elusive extranuclear estrogen receptors in breast cancer. Clin. Cancer Res. 2012, 18, 6–8. [Google Scholar] [CrossRef]
- Acconcia, F.; Ascenzi, P.; Bocedi, A.; Spisni, E.; Tomasi, V.; Trentalance, A.; Visca, P.; Marino, M. Palmitoylation-dependent oestrogen receptor alpha membrane localization: Regulation by 17beta-estradiol. Mol. Biol. Cell 2005, 16, 231–237. [Google Scholar] [CrossRef]
- Marino, M.; Ascenzi, P. Steroid hormone rapid signalling: The pivotal role of S-palmitoylation. IUBMB Life 2006, 58, 716–719. [Google Scholar] [CrossRef]
- Evinger, A.J., 3rd; Levin, E.R. Requirements for oestrogen receptor alpha membrane localization and function. Steroids 2005, 70, 361–363. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, S.B.; Doolan, C.M.; Harvey, B.J. 17beta-oestradiol acutely regulates Cl- secretion in rat distal colonic epithelium. J. Physiol. 2001, 530 Pt 1, 47–54. [Google Scholar] [CrossRef]
- McNamara, B.; Winter, D.C.; Cuffe, J.; Taylor, C.; O’Sullivan, G.C.; Harvey, B.J. Rapid activation of basolateral potassium transport in human colon by oestradiol. Br. J. Pharmacol. 2000, 131, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Doolan, C.M.; Harvey, B.J. A Galphas protein-coupled membrane receptor, distinct from the classical oestrogen receptor, transduces rapid effects of oestradiol on [Ca2+]i in female rat distal colon. Mol. Cell. Endocrinol. 2003, 199, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Arnal, J.F.; Lenfant, F. Segregation of nuclear and membrane-initiated actions of estrogen receptor using genetically modified animals and pharmacological tools. Mol. Cell. Endocrinol. 2022, 539, 111467. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Horkeby, K.; Henning, P.; Wu, J.; Lawenius, L.; Engdahl, C.; Gupta, P.; Movérare-Skrtic, S.; Nilsson, K.H.; Levin, E.; et al. Membrane oestrogen receptor α signalling modulates the sensitivity to estradiol treatment in a dose- and tissue- dependent manner. Sci. Rep. 2023, 13, 9046. [Google Scholar] [CrossRef] [PubMed]
- Stefkovich, M.L.; Arao, Y.; Hamilton, K.J.; Korach, K.S. Experimental models for evaluating non-genomic oestrogen signalling. Steroids 2018, 133, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Pappas, T.C.; Gametchu, B.; Watson, C.S. Membrane oestrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995, 9, 404–410. [Google Scholar] [CrossRef]
- Harrington, W.R.; Kim, S.H.; Funk, C.C.; Madak-Erdogan, Z.; Schiff, R.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S. Oestrogen dendrimer conjugates that preferentially activate extranuclear, nongenomic versus genomic pathways of oestrogen action. Mol. Endocrinol. 2006, 20, 491–502. [Google Scholar] [CrossRef]
- Pedram, A.; Razandi, M.; Kim, J.K.; O’Mahony, F.; Lee, E.Y.; Luderer, U.; Levin, E.R. Developmental phenotype of a membrane only oestrogen receptor alpha [MOER] mouse. J. Biol. Chem. 2009, 284, 3488–3495. [Google Scholar] [CrossRef]
- Adlanmerini, M.; Solinhac, R.; Abot, A.; Fabre, A.; Raymond-Letron, I.; Guihot, A.-L.; Boudou, F.; Sautier, L.; Vessières, E.; Kim, S.H.; et al. Mutation of the palmitoylation site of oestrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc. Natl. Acad. Sci. USA 2014, 111, E283–E290. [Google Scholar] [CrossRef]
- Abot, A.; Fontaine, C.; Buscato, M.; Solinhac, R.; Flouriot, G.; Fabre, A.; Drougard, A.; Rajan, S.; Laine, M.; Milon, A.; et al. The uterine and vascular actions of estetrol delineate a distinctive profile of oestrogen receptor α modulation, uncoupling nuclear and membrane activation. EMBO Mol. Med. 2014, 6, 1328–1346. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Rapid signalling by steroid receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1425–R1430. [Google Scholar] [CrossRef]
- Acconcia, F.; Fiocchetti, M.; Busonero, C.; Fernandez, V.S.; Montalesi, E.; Cipolletti, M.; Pallottini, V.; Marino, M. The extra-nuclear interactome of the oestrogen receptors: Implications for physiological functions. Mol. Cell. Endocrinol. 2021, 538, 111452. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front Endocrinol (Lausanne). Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.C.; Taylor, C.; CO’Sullivan, G.; Harvey, B.J. Mitogenic effects of oestrogen mediated by a non-genomic receptor in human colon. Br. J. Surg. 2000, 87, 1684–1689. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Salazar, J.E.; Posadas-Rodríguez, P.; Lazzarini-Lechuga, R.C.; Luna-López, A.; Zentella-Dehesa, A.; Gómez-Quiroz, L.E.; Königsberg, M.; Domínguez-Gómez, G.; Damián-Matsumura, P. Membrane-Initiated Estradiol Signalling of Epithelial-Mesenchymal Transition-Associated Mechanisms Through Regulation of Tight Junctions in Human Breast Cancer Cells. Horm. Cancer 2014, 5, 161–173. [Google Scholar] [CrossRef]
- Sabouni, E.; Nejad, M.M.; Mojtabavi, S.; Khoshdooz, S.; Mojtabavi, M.; Nadafzadeh, N.; Nikpanjeh, N.; Mirzaei, S.; Hashemi, M.; Aref, A.R.; et al. Unraveling the function of epithelial-mesenchymal transition [EMT] in colorectal cancer: Metastasis, therapy response, and revisiting molecular pathways. Biomed. Pharmacother. 2023, 160, 114395. [Google Scholar] [CrossRef]
- Wang, M.; Deng, C.; Yang, C.; Yan, M.; Lu, H.; Zhang, Y.; Liu, H.; Tong, Z.; Ma, J.; Wang, J.; et al. Unraveling temporal and spatial biomarkers of epithelial-mesenchymal transition in colorectal cancer: Insights into the crucial role of immunosuppressive cells. J. Transl. Med. 2023, 21, 794. [Google Scholar] [CrossRef]
- Marino, M.; Ascenzi, P. Membrane association of oestrogen receptor alpha and beta influences 17beta-estradiol-mediated cancer cell proliferation. Steroids 2008, 73, 853–858. [Google Scholar] [CrossRef]
- Galluzzo, P.; Caiazza, F.; Moreno, S.; Marino, M. Role of ERbeta palmitoylation in the inhibition of human colon cancer cell proliferation. Endocr. Relat. Cancer 2007, 14, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Razandi, M.; Pedram, A.; Greene, G.L.; Levin, E.R. Cell membrane and nuclear oestrogen receptors [ERs] originate from a single transcript: Studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol. Endocrinol. 1999, 13, 307–319. [Google Scholar] [PubMed]
- Marino, M.; Galluzzo, P.; Leone, S.; Acconcia, F.; Ascenzi, P. Nitric oxide impairs the 17β-estradiol-induced apoptosis in human colon adenocarcinoma cells. Endocr. Relat. Cancer 2006, 13, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Acconcia, F.; Totta, P.; Ogawa, S.; Cardillo, I.; Inoue, S.; Leone, S.; Trentalance, A.; Muramatsu, M.; Marino, M. Survival versus apoptotic 17beta-estradiol effect: Role of ER alpha and ER beta activated non-genomic signalling. J. Cell. Physiol. 2005, 203, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Cui, H. Oestrogen Receptor Alpha Splice Variants, Post-Translational Modifications, and Their Physiological Functions. Cells 2023, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Langdon, S.P. Oestrogen Receptor Signalling in Cancer. Cancers 2020, 12, 2744. [Google Scholar] [CrossRef]
- Resnick, E.M.; Schreihofer, D.A.; Periasamy, A.; Shupnik, M.A. Truncated oestrogen receptor product-1 suppresses oestrogen receptor transactivation by dimerization with oestrogen receptors alpha and beta. J. Biol. Chem. 2000, 275, 7158–7166. [Google Scholar] [CrossRef]
- Notas, G.; Panagiotopoulos, A.; Vamvoukaki, R.; Kalyvianaki, K.; Kiagiadaki, F.; Deli, A.; Kampa, M.; Castanas, E. ERα36-GPER1 Collaboration Inhibits TLR4/NFκB-Induced Pro-Inflammatory Activity in Breast Cancer Cells. Int. J. Mol. Sci. 2021, 22, 7603. [Google Scholar] [CrossRef]
- Romano, S.N.; Gorelick, D.A. Crosstalk between nuclear and G protein-coupled estrogen receptors. Gen. Comp. Endocrinol. 2018, 261, 190–197. [Google Scholar] [CrossRef]
- Pagano, M.T.; Ortona, E.; Dupuis, M.L. A Role for Oestrogen Receptor alpha36 in Cancer Progression. Front. Endocrinol. 2020, 11, 506. [Google Scholar] [CrossRef]
- Deng, H.; Huang, X.; Fan, J.; Wang, L.; Xia, Q.; Yang, X.; Wang, Z.; Liu, L. A variant of oestrogen receptor-alpha, ER-alpha36 is expressed in human gastric cancer and is highly correlated with lymph node metastasis. Oncol Rep. 2010, 24, 171–176. [Google Scholar] [PubMed]
- Thomas, C.; Gustafsson, J.Å. Oestrogen receptor mutations and functional consequences for breast cancer. Trends Endocrinol. Metab. 2015, 26, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Teng, R.; Wang, Q.; Zhang, X.; Wang, H.; Wang, Z.; Cao, J.; Teng, L. Transcriptional analysis of oestrogen receptor alpha variant mRNAs in colorectal cancers and their matched normal colorectal tissues. J. Steroid Biochem. Mol. Biol. 2008, 112, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular oestrogen receptor mediates rapid cell signalling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.J.; Thomas, P. Minireview: G Protein-Coupled Oestrogen Receptor-1, GPER-1: Its Mechanism of Action and Role in Female Reproductive Cancer, Renal and Vascular Physiology. Endocrinology 2012, 153, 2953–2962. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Pang, Y.; Filardo, E.J.; Dong, J. Identity of an oestrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology 2004, 164, 624–632. [Google Scholar]
- Qiu, Y.A.; Xiong, J.; Yu, T. Role of G Protein-Coupled Oestrogen Receptor in Digestive System Carcinomas: A Minireview. Onco Targets Ther. 2021, 14, 2611–2622. [Google Scholar] [CrossRef]
- Hall, K.A.; Filardo, E.J. The G Protein-Coupled Oestrogen Receptor (GPER): A Critical Therapeutic Target for Cancer. Cells 2023, 12, 2460. [Google Scholar] [CrossRef]
- Barton, M.; Filardo, E.J.; Lolait, S.J.; Thomas, P.; Maggiolini, M.; Prossnitz, E.R. Twenty years of the G protein-coupled oestrogen receptor GPER: Historical and personal perspectives. J. Steroid Biochem. Mol. Biol. 2018, 176, 4–15. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Barton, M. The G protein-coupled oestrogen receptor GPER in health and disease: An update. Nat. Rev. Endocrinol. 2023, 19, 407–424. [Google Scholar] [CrossRef]
- Levin, E.R. G protein-coupled receptor 30: Oestrogen receptor or collaborator? Endocrinology 2009, 150, 1563–1565. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, D. Does GPER Really Function as a G Protein-Coupled Oestrogen Receptor in vivo? Front Endocrinol. 2020, 11, 148. [Google Scholar] [CrossRef]
- Jacenik, D.; Krajewska, W.M. Significance of G Protein-Coupled Oestrogen Receptor in the Pathophysiology of Irritable Bowel Syndrome, Inflammatory Bowel Diseases and Colorectal Cancer. Front. Endocrinol. 2020, 11, 390. [Google Scholar] [CrossRef] [PubMed]
- Girgert, R.; Emons, G.; Gründker, C. Oestrogen Signalling in ERα-Negative Breast Cancer: ERβ and GPER. Front. Endocrinol. 2019, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Bustos, V.; Nolan, Á.M.; Nijhuis, A.; Harvey, H.; Parker, A.; Poulsom, R.; McBryan, J.; Thomas, W.; Silver, A.; Harvey, B.J. GPER mediates differential effects of oestrogen on colon cancer cell proliferation and migration under normoxic and hypoxic conditions. Oncotarget 2017, 8, 84258–84275. [Google Scholar] [CrossRef] [PubMed]
- Jacenik, D.; Cygankiewicz, A.I.; Mokrowiecka, A.; Małecka-Panas, E.; Fichna, J.; Krajewska, W.M. Sex- and Age-Related Oestrogen Signalling Alteration in Inflammatory Bowel Diseases: Modulatory Role of Oestrogen Receptors. Int. J. Mol. Sci. 2019, 20, 3175. [Google Scholar] [CrossRef] [PubMed]
- van der Giessen, J.; van der Woude, C.J.; Peppelenbosch, M.P.; Fuhler, G.M. A Direct Effect of Sex Hormones on Epithelial Barrier Function in Inflammatory Bowel Disease Models. Cells 2019, 8, 261. [Google Scholar] [CrossRef]
- Rouhimoghadam, M.; Lu, A.S.; Salem, A.K.; Filardo, E.J. Therapeutic Perspectives on the Modulation of G-Protein Coupled Oestrogen Receptor, GPER, Function. Front. Endocrinol. 2020, 11, 591217. [Google Scholar] [CrossRef]
- Skrzypczak, M.; Goryca, K.; Rubel, T.; Paziewska, A.; Mikula, M.; Jarosz, D.; Pachlewski, J.; Oledzki, J.; Ostrowsk, J. Modeling oncogenic signalling in colon tumours by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS ONE 2010, 5, 12, Erratum in PLoS ONE 2010, 5, e13091. [Google Scholar] [CrossRef]
- Jung, J. Role of G Protein-Coupled Oestrogen Receptor in Cancer Progression. Toxicol. Res. 2019, 35, 209–214. [Google Scholar] [CrossRef]
- Pepermans, R.A.; Sharma, G.; Prossnitz, E.R. G Protein-Coupled Oestrogen Receptor in Cancer and Stromal Cells: Functions and Novel Therapeutic Perspectives. Cells 2021, 10, 672. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Z.; Jiang, G.; Zhou, Y.; Yang, X.; Huang, H.; Liu, H.; Du, J.; Wang, H. Epigenetic down regulation of G protein-coupled oestrogen receptor [GPER] functions as a tumour suppressor in colorectal cancer. Mol. Cancer 2017, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Gilligan, L.C.; Rahman, H.P.; Hewitt, A.M.; Sitch, A.J.; Gondal, A.; Arvaniti, A.; Taylor, A.E.; Read, M.L.; Morton, D.G.; Foster, P.A. Oestrogen Activation by Steroid Sulfatase Increases Colorectal Cancer Proliferation via GPER. J. Clin. Endocrinol. Metab. 2017, 102, 4435–4447. [Google Scholar] [CrossRef] [PubMed]
- Lo, E.K.K.; Lee, J.C.-Y.; Turner, P.C.; El-Nezami, H. Low dose of zearalenone elevated colon cancer cell growth through G protein-coupled estrogenic receptor. Sci. Rep. 2021, 11, 7403. [Google Scholar] [CrossRef] [PubMed]
- Bühler, M.; Fahrländer, J.; Sauter, A.; Becker, M.; Wistorf, E.; Steinfath, M.; Stolz, A. GPER1 links estrogens to centrosome amplification and chromosomal instability in human colon cells. Life Sci. Alliance 2022, 6, e202201499. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, E.M.; Sims, A.H.; Maggiolini, M.; Sotgia, F.; Lisanti, M.P.; Clarke, R.B. GPER mediates the angiocrine actions induced by IGF1 through the HIF-1α/VEGF pathway in the breast tumour microenvironment. Breast Cancer Res. 2017, 19, 129. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ma, J.; Wang, X.; Wang, X.; Fang, W.; Sun, J.; Li, Z.; Liu, J. The Role of G Protein-Coupled Estrogen Receptor (GPER) in Vascular Pathology and Physiology. Biomolecules 2023, 13, 1410. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L. Hypoxia--a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002, 2, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Hou, M.; Guan, Y.; Jiang, M.; Yang, Y.; Gou, H. Expression of HIF-1alpha and VEGF in colorectal cancer: Association with clinical outcomes and prognostic implications. BMC Cancer 2009, 9, 432. [Google Scholar] [CrossRef]
- Beggs, A.D.; Domingo, E.; McGregor, M.; Presz, M.; Johnstone, E.; Midgley, R.; Kerr, D.; Oukrif, D.; Novelli, M.; Abulafi, M.; et al. Loss of expression of the double strand break repair protein ATM is associated with worse prognosis in colorectal cancer and loss of Ku70 expression is associated with CIN. Oncotarget 2012, 3, 1348–1355. [Google Scholar] [CrossRef]
- El-Khatib, M.; Geara, F.; Haddadin, M.; Gali-Muhtasib, H. Cell death by the quinoxaline dioxide DCQ in human colon cancer cells is enhanced under hypoxia and is independent of p53 and p21. Radiat. Oncol. 2010, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Nusse, R. Wnt/β-catenin signalling and disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Schatoff, E.M.; Leach, B.I.; Dow, L.E. Wnt Signalling and Colorectal Cancer. Curr. Colorectal. Cancer Rep. 2017, 13, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ming, T.; Tang, S.; Ren, S.; Yang, H.; Liu, M.; Tao, Q.; Xu, H. Wnt signalling in colorectal cancer: Pathogenic role and therapeutic target. Mol. Cancer 2022, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Sontz, R.A.; Vance, M.J.; Morris, J.M.; Sheriff, S.; Zhu, S.; Duan, S.; Zeng, J.; Koeppe, E.; Pandey, R.; et al. High-fat diet plus HNF1A variant promotes polyps by activating β-catenin in early-onset colorectal cancer. JCI Insight 2023, 8, e167163. [Google Scholar] [CrossRef]
- Kouzmenko, A.P.; Takeyama, K.-I.; Ito, S.; Furutani, T.; Sawatsubashi, S.; Maki, A.; Suzuki, E.; Kawasaki, Y.; Akiyama, T.; Tabata, T.; et al. Wnt/beta-catenin and oestrogen signalling converge in vivo. J. Biol. Chem. 2004, 279, 40255–40258. [Google Scholar] [CrossRef]
- Xiong, W.; Zhang, L.; Yu, L.; Xie, W.; Man, Y.; Xiong, Y.; Liu, H.; Liu, Y. Estradiol promotes cells invasion by activating β-catenin signalling pathway in endometriosis. Reproduction 2015, 150, 507–516. [Google Scholar] [CrossRef]
- Hou, X.; Tan, Y.; Li, M.; Dey, S.K.; Das, S.K. Canonical Wnt Signalling Is Critical to Oestrogen-Mediated Uterine Growth. Mol. Endocrinol. 2004, 18, 3035–3049. [Google Scholar] [CrossRef]
- Bhat, M.; Pasini, E.; Pastrello, C.; Angeli, M.; Baciu, C.; Abovsky, M.; Coffee, A.; Adeyi, O.; Kotlyar, M.; Jurisica, I. Oestrogen Receptor 1 Inhibition of Wnt/β-Catenin Signalling Contributes to Sex Differences in Hepatocarcinogenesis. Front. Oncol. 2021, 11, 777834. [Google Scholar] [CrossRef]
- Abbott, G.W. Biology of the KCNQ1 Potassium Channel. New J. Sci. 2014, 2014, 237431. [Google Scholar] [CrossRef]
- Schroeder, B.C.; Waldegger, S.; Fehr, S.; Bleich, M.; Warth, R.; Greger, R.; Jentsch, T.J. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature 2000, 403, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Than, B.L.; Goos, J.A.; Sarver, A.L.; O’Sullivan, M.G.; Rod, A.; Starr, T.K.; Fijneman, R.J.; Meijer, G.A.; Zhao, L.; Zhang, Y.; et al. The role of KCNQ1 in mouse and human gastrointestinal cancers. Oncogene 2014, 33, 3861–3868. [Google Scholar] [CrossRef] [PubMed]
- Rapetti-Mauss, R.; Bustos, V.; Thomas, W.; McBryan, J.; Harvey, H.; Lajczak, N.; Madden, S.F.; Pellissier, B.; Borgese, F.; Soriani, O.; et al. Bidirectional KCNQ1:β-catenin interaction drives colorectal cancer cell differentiation. Proc. Natl. Acad. Sci. USA 2017, 114, 4159–4164. [Google Scholar] [CrossRef] [PubMed]
- Shorthouse, D.; Zhuang, L.; Rahrmann, E.P.; Kosmidou, C.; Rahrmann, K.W.; Hall, M.; Greenwood, B.; Devonshire, G.; Gilbertson, R.J.; Fitzgerald, R.C.; et al. KCNQ potassium channels modulate Wnt activity in gastro-oesophageal adenocarcinomas. Life Sci. Alliance 2023, 6, e202302124. [Google Scholar] [CrossRef] [PubMed]
- Alzamora, R.; O’Mahony, F.; Bustos, V.; Rapetti-Mauss, R.; Urbach, V.; Cid, L.P.; Sepúlveda, F.V.; Harvey, B.J. Sexual dimorphism and oestrogen regulation of KCNE3 expression modulates the functional properties of KCNQ1 K⁺ channels. J. Physiol. 2011, 589 Pt 21, 5091–5107. [Google Scholar] [CrossRef]
- Rapetti-Mauss, R.; O’Mahony, F.; Sepulveda, F.V.; Urbach, V.; Harvey, B.J. Oestrogen promotes KCNQ1 potassium channel endocytosis and post-endocytic trafficking in colonic epithelium. J. Physiol. 2013, 591, 2813–2831. [Google Scholar] [CrossRef] [PubMed]
- Rapetti-Mauss, R.; Borgese, F.; Harvey, B.J.; Soriani, O. KCNQ1: A new regulator of the epithelio-mesenchymal transition in colorectal cancers. Med. Sci. 2018, 34, 21–24. [Google Scholar] [CrossRef]
- Rapetti-Mauss, R.; Berenguier, C.; Allegrini, B.; Soriani, O. Interplay Between Ion Channels and the Wnt/β-Catenin Signalling Pathway in Cancers. Front Pharmacol. 2020, 11, 525020. [Google Scholar] [CrossRef]
- Hu, T.; Li, C. Convergence between Wnt-β-catenin and EGFR signalling in cancer. Mol. Cancer 2010, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Luo, K. Signalling Cross Talk between TGF-β/Smad and Other Signalling Pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef] [PubMed]
- Abancens, M.; Harvey, B.J.; McBryan, J. GPER Agonist G1 Prevents Wnt-Induced JUN Upregulation in HT29 Colorectal Cancer Cells. Int. J. Mol. Sci. 2022, 23, 12581. [Google Scholar] [CrossRef]
- Hasáková, K.; Bezakova, J.; Vician, M.; Reis, R.; Zeman, M.; Herichova, I. Gender-dependent expression of leading and passenger strand of miR-21 and miR-16 in human colorectal cancer and adjacent colonic tissues. Physiol. Res. 2017, 66 (Suppl. 4), S575–S582. [Google Scholar] [CrossRef] [PubMed]
- Hamfjord, J.; Stangeland, A.M.; Hughes, T.; Skrede, M.L.; Tveit, K.M.; Ikdahl, T.; Kure, E.H. Differential expression of miRNAs in colorectal cancer: Comparison of paired tumour tissue and adjacent normal mucosa using high-throughput sequencing. PLoS ONE 2012, 7, e34150. [Google Scholar] [CrossRef] [PubMed]
- Massaro, C.; Safadeh, E.; Sgueglia, G.; Stunnenberg, H.G.; Altucci, L.; Dell’Aversana, C. MicroRNA-Assisted Hormone Cell Signalling in Colorectal Cancer Resistance. Cells 2020, 10, 39. [Google Scholar] [CrossRef] [PubMed]
- Herichova, I.; Reis, R.; Hasakova, K.; Vician, M. Downregulation of miR-30c-5p expression in colorectal cancer tissue is sex-dependent. Physiol. Res. 2020, 69 (Suppl. 3), S479–S487. [Google Scholar] [CrossRef]
- Mehrgou, A.; Ebadollahi, S.; Seidi, K.; Ayoubi-Joshaghani, M.H.; Ahmadieh Yazdi, A.; Zare, P.; Jaymand, M.; Jahanban-Esfahlan, R. Roles of miRNAs in Colorectal Cancer: Therapeutic Implications and Clinical Opportunities. Adv. Pharm. Bull. 2021, 11, 233–247. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Men’s Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Markle, J.G.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Oestrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H.; Kim, N.; Nam, R.H.; Choi, S.I.; Lee, H.N.; Surh, Y.J. 17β-Estradiol supplementation changes gut microbiota diversity in intact and colorectal cancer-induced ICR male mice. Sci. Rep. 2020, 10, 12283. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Alcoholado, L.; Ramos-Molina, B.; Otero, A.; Laborda-Illanes, A.; Ordóñez, R.; Medina, J.A.; Gómez-Millán, J.; Queipo-Ortuño, M.I. The Role of the Gut Microbiome in Colorectal Cancer Development and Therapy Response. Cancers 2020, 12, 1406. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef]
- Roshan, M.H.; Tambo, A.; Pace, N.P. The role of testosterone in colorectal carcinoma: Pathomechanisms and open questions. EPMA J. 2016, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lan, Z.; Liao, W.; Horner, J.W.; Xu, X.; Liu, J.; Yoshihama, Y.; Jiang, S.; Shim, H.S.; Slotnik, M.; et al. Histone demethylase KDM5D upregulation drives sex differences in colon cancer. Nature 2023, 619, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Ledford, H. How the Y chromosome makes some cancers more deadly for men. Nature 2023, 618, 898. [Google Scholar] [CrossRef] [PubMed]
- Amos-Landgraf, J.M.; Heijmans, J.; Wielenga, M.C.B.; Dunkin, E.; Krentz, K.J.; Clipson, L.; Ederveen, A.G.; Groothuis, P.G.; Mosselman, S.; Muncan, V.; et al. Sex disparity in colonic adenoma genesis involves promotion by male hormones, not protection by female hormones. Proc. Natl. Acad. Sci. USA 2014, 111, 16514–16519. [Google Scholar] [CrossRef]
- Harbs, J.; Rinaldi, S.; Gicquiau, A.; Keski-Rahkonen, P.; Mori, N.; Liu, X.; Kaaks, R.; Katzke, V.; Schulze, M.B.; Agnoli, C.; et al. Circulating Sex Hormone Levels and Colon Cancer Risk in Men: A Nested Case-Control Study and Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2022, 31, 793–803. [Google Scholar] [CrossRef]
- Slattery, M.L.; Sweeney, C.; Murtaugh, M.; Ni Ma, K.; Wolff, R.K.; Potter, J.D.; Caan, B.J.; Samowitz, W. Associations between ERalpha, ERbeta, and AR genotypes and colon and rectal cancer. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2936–2942. [Google Scholar] [CrossRef]
- Catalano, M.G.; Pfeffer, U.; Raineri, M.; Ferro, P.; Curto, A.; Capuzzi, P.; Corno, F.; Berta, L.; Fortunati, N. Altered expression of androgen-receptor isoforms in human colon-cancer tissues. Int. J. Cancer 2000, 86, 325–330. [Google Scholar] [CrossRef]
- Gu, S.; Papadopoulou, N.; Nasir, O.; Föller, M.; Alevizopoulos, K.; Lang, F.; Stournaras, C. Activation of membrane androgen receptors in colon cancer inhibits the prosurvival signals Akt/Bad in vitro and in vivo and blocks migration via vinculin/actin signalling. Mol. Med. 2011, 17, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.S.; Chlebowski, R.T.; Wactawski-Wende, J.; Johnson, K.C.; Muskovitz, A.; Kato, I.; Young, A.; Hubbell, F.A.; Prentice, R.L. Estrogen plus progestin and colorectal cancer incidence and mortality. J. Clin. Oncol. 2012, 30, 3983–3990. [Google Scholar] [CrossRef] [PubMed]
- Patman, G. Colorectal cancer: Male hormones increase the incidence of colonic adenomas. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Krasanakis, T.; Nikolouzakis, T.K.; Sgantzos, M.; Mariolis-Sapsakos, T.; Souglakos, J.; Spandidos, D.A.; Tsitsimpikou, C.; Tsatsakis, A.; Tsiaoussis, J. Role of anabolic agents in colorectal carcinogenesis: Myths and realities (Review). Oncol. Rep. 2019, 42, 2228–2244. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Young, M.-J.; Chang, H.-P.; Liu, C.-Y.; Lee, C.-C.; Tseng, Y.-L.; Wang, Y.-C.; Chang, W.-C.; Hung, J.-J. Estradiol-mediated inhibition of DNMT1 decreases p53 expression to induce M2-macrophage polarization in lung cancer progression. Oncogenesis 2022, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Fan, W.; Gao, X.; Huang, K.; Ding, C.; Ma, G.; Yan, L.; Song, S. Oestrogen receptor alpha regulates the Wnt/β-catenin signalling pathway in colon cancer by targeting the NOD-like receptors. Cell Signal. Cell. Signal. 2019, 61, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, K.; Nguyen-Vu, T.; Kalasekar, S.M.; Pontén, F.; Gustafsson, J.Å.; Williams, C. Oestrogen receptor β expression induces changes in the microRNA pool in human colon cancer cells. Carcinogenesis 2013, 34, 1431–1441. [Google Scholar] [CrossRef]
- Lu, M.H.; Huang, C.C.; Pan, M.R.; Chen, H.H.; Hung, W.C. Prospero homeobox 1 promotes epithelial-mesenchymal transition in colon cancer cells by inhibiting E-cadherin via miR-9. Clin. Cancer Res. 2012, 18, 6416–6425. [Google Scholar] [CrossRef]
- Hankey, W.; Frankel, W.L.; Groden, J. Functions of the APC tumour suppressor protein dependent and independent of canonical WNT signalling: Implications for therapeutic targeting. Cancer Metastasis Rev. 2018, 37, 159–172. [Google Scholar] [CrossRef]
- Xiao, L.; Luo, Y.; Tai, R.; Zhang, N. Oestrogen receptor β suppresses inflammation and the progression of prostate cancer. Mol. Med. Rep. 2019, 19, 3555–3563. [Google Scholar] [CrossRef] [PubMed]
- Shivers, K.Y.; Amador, N.; Abrams, L.; Hunter, D.; Jenab, S.; Quiñones-Jenab, V. Oestrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine 2015, 72, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.M.; Chen, Y.C.; Jiang, M.C. 17 beta-estradiol inhibits tumour necrosis factor-alpha-induced nuclear factor-kappa B activation by increasing nuclear factor-kappa B p105 level in MCF-7 breast cancer cells. Biochem. Biophys. Res. Commun. 2000, 279, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Oparil, S.; Yu, H.; Gong, K.; Feng, W.; Black, J.; Chen, Y.F.; Nozell, S. Oestrogen modulates NFκB signalling by enhancing IκBα levels and blocking p65 binding at the promoters of inflammatory genes via oestrogen receptor-β. PLoS ONE 2012, 7, e36890. [Google Scholar] [CrossRef] [PubMed]
- Xing, D.; Feng, W.; Miller, A.P.; Weathington, N.M.; Chen, Y.F.; Novak, L.; Blalock, J.E.; Oparil, S. Estrogen modulates TNF-alpha-induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H2607-12. [Google Scholar] [CrossRef] [PubMed]
- Rainer, H. Straub, The Complex Role of Estrogens in Inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef]
- Karpuzoglu-Sahin, E.; Hissong, B.D.; Ansar Ahmed, S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J. Reprod. Immunol. 2001, 52, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.; Flanagan, K. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Syrett, C.M.; Anguera, M.C. When the balance is broken: X-linked gene dosage from two X chromosomes and female-biased autoimmunity. J. Leukoc. Biol. 2019, 106, 919–932. [Google Scholar] [CrossRef]
- Sarmiento, L.; Svensson, J.; Barchetta, I.; Giwercman, A.; Cilio, C.M. Copy number of the X-linked genes TLR7 and CD40L influences innate and adaptive immune responses. Scand. J. Immunol. 2019, 90, e12776. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef]
- Jiang, C.; Xu, F.; Yi, D.; Jiang, B.; Wang, R.; Wu, L.; Ding, H.; Qin, J.; Lee, Y.; Sang, J.; et al. Testosterone promotes the migration, invasion and EMT process of papillary thyroid carcinoma by up-regulating Tnnt1. J. Endocrinol. Investig. 2023, 1–18. [Google Scholar] [CrossRef]
- Nedresky, D.; Singh, G. Physiology, Luteinizing Hormone. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Cohen, P.G. Aromatase, adiposity, aging and disease. The hypogonadal-metabolic-atherogenic-disease and aging connection. Med. Hypotheses 2001, 56, 702–708. [Google Scholar] [CrossRef]
- Mahbub, A.A. Therapeutic Strategies and Potential Actions of Female Sex Steroid Hormones and Their Receptors in Colon Cancer Based on Preclinical Studies. Life 2022, 12, 605. [Google Scholar] [CrossRef]
- Andrei, P.; Battuello, P.; Grasso, G.; Rovera, E.; Tesio, N.; Bardelli, A. Integrated approaches for precision oncology in colorectal cancer: The more you know, the better. Semin. Cancer Biol. 2022, 84, 199–213. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey, B.J.; Harvey, H.M. Sex Differences in Colon Cancer: Genomic and Nongenomic Signalling of Oestrogen. Genes 2023, 14, 2225. https://doi.org/10.3390/genes14122225
Harvey BJ, Harvey HM. Sex Differences in Colon Cancer: Genomic and Nongenomic Signalling of Oestrogen. Genes. 2023; 14(12):2225. https://doi.org/10.3390/genes14122225
Chicago/Turabian StyleHarvey, Brian J., and Harry M. Harvey. 2023. "Sex Differences in Colon Cancer: Genomic and Nongenomic Signalling of Oestrogen" Genes 14, no. 12: 2225. https://doi.org/10.3390/genes14122225