An RNA Interference (RNAi) Toolkit and Its Utility for Functional Genetic Analysis of Leishmania (Viannia)
Abstract
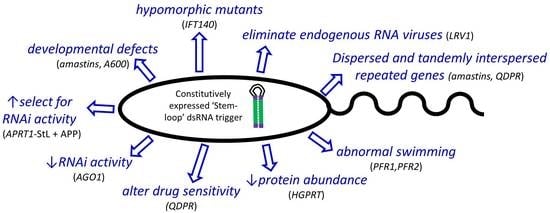
:1. Introduction
2. Materials and Methods
2.1. Leishmania Strains and Parasite Culture
2.2. Leishmania Transfection
2.3. Western-Blot Analysis
2.4. Quininoid Dihydropteridine Reductase Assay
2.5. Luciferase Assay
2.6. Transmission Electron Microscopy
2.7. Construction of the Integrating pIR-GW Destination Vectors Facilitating Generation of StL Constructs Using Gateway Site Specific Recombinase
2.8. Generation of Target Stem-Loop (StL) Constructs for RNAi
3. Results
3.1. Rapid Generation of ‘Stem-Loop’ Constructs as RNAi Triggers
3.2. Testing the Effect of Stem Length on RNAi Activity Using L. braziliensis HGPRT-StL and Luciferase-StL Series Constructs
3.3. StL-Mediated Specific RNAi of the Metabolic Target Quinonoid Dihydropteridine Reductase (QDPR) Interspersed within a Tandem Repeated Gene Array
3.4. RNAi of an L. braziliensis Gene Important for Amastigote Replication
3.5. Targeting Essential Genes of the L. braziliensis Intraflagellar Transport (IFT) Pathway
3.6. Systematic Generation of Hypomorphic Mutants by Exploiting the Stem Length-Dependency of RNAi in L. braziliensis
3.7. A Negative Selection System for Enhancement of RNAi Activity in L. braziliensis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Velez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Pigott, D.M.; Bhatt, S.; Golding, N.; Duda, K.A.; Battle, K.E.; Brady, O.J.; Messina, J.P.; Balard, Y.; Bastien, P.; Pratlong, F.; et al. Global distribution maps of the leishmaniases. Elife 2014, 3, e28051. [Google Scholar] [CrossRef] [PubMed]
- Banuls, A.L.; Bastien, P.; Pomares, C.; Arevalo, J.; Fisa, R.; Hide, M. Clinical pleiomorphism in human leishmaniases, with special mention of asymptomatic infection. Clin Microbiol. Infect. 2011, 17, 1451–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herwaldt, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef] [Green Version]
- Mannan, S.B.; Elhadad, H.; Loc, T.T.H.; Sadik, M.; Mohamed, M.Y.F.; Nam, N.H.; Thuong, N.D.; Hoang-Trong, B.L.; Duc, N.T.M.; Hoang, A.N.; et al. Prevalence and associated factors of asymptomatic leishmaniasis: A systematic review and meta-analysis. Parasitol. Int. 2021, 81, 102229. [Google Scholar] [CrossRef] [PubMed]
- Banuls, A.L.; Hide, M.; Prugnolle, F. Leishmania and the leishmaniases: A parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. Adv. Parasitol. 2007, 64, 1–109. [Google Scholar] [PubMed]
- Lainson, R.; Shaw, J.J. Evolution, Classification and Geographical Distribution of LEISHMANIA; Killick-Kendrick, R., Peters, W., Eds.; The Leishmaniasis; Academic Press: London, UK, 1987; pp. 1–120. [Google Scholar]
- McGwire, B.S.; Satoskar, A.R. Leishmaniasis: Clinical syndromes and treatment. QJM 2014, 107, 7–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartley, M.A.; Ronet, C.; Zangger, H.; Beverley, S.M.; Fasel, N. Leishmania RNA virus: When the host pays the toll. Front. Cell Infect. Microbiol. 2012, 2, 99. [Google Scholar] [CrossRef] [Green Version]
- Lye, L.F.; Owens, K.; Shi, H.; Murta, S.M.; Vieira, A.C.; Turco, S.J.; Tschudi, C.; Ullu, E.; Beverley, S.M. Retention and loss of RNA interference pathways in trypanosomatid protozoans. PLoS Pathog. 2010, 6, e1001161. [Google Scholar] [CrossRef] [Green Version]
- Guerra, J.A.; Prestes, S.R.; Silveira, H.; Coelho, L.I.; Gama, P.; Moura, A.; Amato, V.; Barbosa, M.G.; Ferreira, L.C. Mucosal Leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl. Trop. Dis. 2011, 5, e980. [Google Scholar] [CrossRef]
- Cerutti, H.; Casas-Mollano, J.A. On the origin and functions of RNA-mediated silencing: From protists to man. Curr. Genet. 2006, 50, 81–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, H.; Tschudi, C.; Gull, K.; Ullu, E. Double-stranded RNA induces mRNA degradation in Trypanosoma brucei. Proc. Natl. Acad. Sci. USA 1998, 95, 14687–14692. [Google Scholar] [CrossRef] [Green Version]
- Djikeng, A.; Shen, S.; Tschudi, C.; Ullu, E. Analysis of gene function in Trypanosoma brucei using RNA interference. Methods Mol. Biol. 2004, 265, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Wang, Z.; Drew, M.E.; Englund, P.T. Glycolysis modulates trypanosome glycoprotein expression as revealed by an RNAi library. EMBO J. 2002, 21, 4429–4438. [Google Scholar] [CrossRef] [Green Version]
- Alsford, S.; Turner, D.J.; Obado, S.O.; Sanchez-Flores, A.; Glover, L.; Berriman, M.; Hertz-Fowler, C.; Horn, D. High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome. Res. 2011, 21, 915–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beverley, S.M. Protozomics: Trypanosomatid parasite genetics comes of age. Nat. Rev. Genet. 2003, 4, 11–19. [Google Scholar] [CrossRef]
- Kolev, N.G.; Tschudi, C.; Ullu, E. RNA interference in protozoan parasites: Achievements and challenges. Eukaryot. Cell 2011, 10, 1156–1163. [Google Scholar] [CrossRef] [Green Version]
- Brettmann, E.A.; Shaik, J.S.; Zangger, H.; Lye, L.F.; Kuhlmann, F.M.; Akopyants, N.S.; Oschwald, D.M.; Owens, K.L.; Hickerson, S.M.; Ronet, C.; et al. Tilting the balance between RNA interference and replication eradicates Leishmania RNA virus 1 and mitigates the inflammatory response. Proc. Natl. Acad. Sci. USA 2016, 113, 11998–12005. [Google Scholar] [CrossRef] [Green Version]
- Adli, M. The CRISPR tool kit for genome editing and beyond. Nat. Commun. 2018, 9, 1911. [Google Scholar] [CrossRef] [Green Version]
- de Paiva, R.M.; Grazielle-Silva, V.; Cardoso, M.S.; Nakagaki, B.N.; Mendonca-Neto, R.P.; Canavaci, A.M.; Souza Melo, N.; Martinelli, P.M.; Fernandes, A.P.; daRocha, W.D.; et al. Amastin Knockdown in Leishmania braziliensis Affects Parasite-Macrophage Interaction and Results in Impaired Viability of Intracellular Amastigotes. PLoS Pathog. 2015, 11, e1005296. [Google Scholar] [CrossRef]
- Kalidas, S.; Li, Q.; Phillips, M.A. A Gateway(R) compatible vector for gene silencing in bloodstream form Trypanosoma brucei. Mol. Biochem. Parasitol. 2011, 178, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peacock, C.S.; Seeger, K.; Harris, D.; Murphy, L.; Ruiz, J.C.; Quail, M.A.; Peters, N.; Adlem, E.; Tivey, A.; Aslett, M.; et al. Comparative genomic analysis of three Leishmania species that cause diverse human disease. Nat. Genet. 2007, 39, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balanco, J.M.; Pral, E.M.; da Silva, S.; Bijovsky, A.T.; Mortara, R.A.; Alfieri, S.C. Axenic cultivation and partial characterization of Leishmania braziliensis amastigote-like stages. Parasitology 1998, 116, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.A.; Beverley, S.M. Improvements in transfection efficiency and tests of RNA interference (RNAi) approaches in the protozoan parasite Leishmania. Mol. Biochem. Parasitol. 2003, 128, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Lye, L.F.; Kang, S.O.; Nosanchuk, J.D.; Casadevall, A.; Beverley, S.M. Phenylalanine hydroxylase (PAH) from the lower eukaryote Leishmania major. Mol. Biochem. Parasitol. 2011, 175, 58–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boitz, J.M.; Ullman, B. Leishmania donovani singly deficient in HGPRT, APRT or XPRT are viable in vitro and within mammalian macrophages. Mol. Biochem. Parasitol. 2006, 148, 24–30. [Google Scholar] [CrossRef]
- Anderson, B.A.; Wong, I.L.; Baugh, L.; Ramasamy, G.; Myler, P.J.; Beverley, S.M. Kinetoplastid-specific histone variant functions are conserved in Leishmania major. Mol. Biochem. Parasitol. 2013, 191, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Lye, L.F.; Cunningham, M.L.; Beverley, S.M. Characterization of quinonoid-dihydropteridine reductase (QDPR) from the lower eukaryote Leishmania major. J. Biol. Chem. 2002, 277, 38245–38253. [Google Scholar] [CrossRef] [Green Version]
- Ong, H.B.; Sienkiewicz, N.; Wyllie, S.; Fairlamb, A.H. Dissecting the Metabolic Roles of Pteridine Reductase 1 in Trypanosoma brucei and Leishmania major. J. Biol. Chem. 2011, 286, 10429–10438. [Google Scholar] [CrossRef] [Green Version]
- Nare, B.; Hardy, L.W.; Beverley, S.M. The roles of pteridine reductase 1 and dihydrofolate reductase-thymidylate synthase in pteridine metabolism in the protozoan parasite Leishmania major. J. Biol. Chem. 1997, 272, 13883–13891. [Google Scholar] [CrossRef]
- Firgaira, F.A.; Cotton, R.G.; Danks, D.M. Isolation and characterization of dihydropteridine reductase from human liver. Biochem. J. 1981, 197, 31–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wesley, S.V.; Liu, Q.; Wielopolska, A.; Ellacott, G.; Smith, N.; Singh, S.; Helliwell, C. Custom knock-outs with hairpin RNA-mediated gene silencing. Methods Mol. Biol. 2003, 236, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Capul, A.A.; Barron, T.; Dobson, D.E.; Turco, S.J.; Beverley, S.M. Two functionally divergent UDP-Gal nucleotide sugar transporters participate in phosphoglycan synthesis in Leishmania major. J. Biol. Chem. 2007, 282, 14006–14017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.; Djikeng, A.; Mark, T.; Wirtz, E.; Tschudi, C.; Ullu, E. Genetic interference in Trypanosoma brucei by heritable and inducible double-stranded RNA. RNA 2000, 6, 1069–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand-Dubief, M.; Kohl, L.; Bastin, P. Efficiency and specificity of RNA interference generated by intra- and intermolecular double stranded RNA in Trypanosoma brucei. Mol. Biochem. Parasitol. 2003, 129, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Ilves, H.; Dallas, A.; Kumar, P.; Shorenstein, J.; Kazakov, S.A.; Johnston, B.H. Minimal-length short hairpin RNAs: The relationship of structure and RNAi activity. RNA 2010, 16, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elbashir, S.M.; Lendeckel, W.; Tuschl, T. RNA interference is mediated by 21- and 22-nucleotide RNAs. Genes Dev. 2001, 15, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardy, L.W.; Matthews, W.; Nare, B.; Beverley, S.M. Biochemical and genetic tests for inhibitors of Leishmania pteridine pathways. Exp. Parasitol. 1997, 87, 157–169. [Google Scholar] [CrossRef]
- Pan, A.A.; Duboise, M.; Eperon, S.; Rivas, L.; Hodgkinson, V.; Traub-Cseko, Y.; McMahon-Pratt, D. Developmental life cycle of Leishmania: Cultivation and characterization of cultured extracellular amastigotes. J. Euk. Microbiol. 1993, 40, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Dias-Lopes, G.; Zabala-Penafiel, A.; de Albuquerque-Melo, B.C.; Souza-Silva, F.; Menaguali do Canto, L.; Cysne-Finkelstein, L.; Alves, C.R. Axenic amastigotes of Leishmania species as a suitable model for in vitro studies. Acta. Trop. 2021, 220, 105956. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.S.; Lynn, M.A.; McMaster, W.R. The Leishmania mexicana A600 genes are functionally required for amastigote replication. Mol. Biochem. Parasitol. 2010, 172, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, H.; Marshall, W.F. Intraflagellar Transport and Ciliary Dynamics. Cold Spring Harb. Perspect Biol. 2017, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atayde, V.D.; Shi, H.; Franklin, J.B.; Carriero, N.; Notton, T.; Lye, L.-F.; Owens, K.; Beverley, S.M.; Tschudi, C.; Ullu, E. The structure and repertoire of small interfering RNAs in Leishmania (Viannia) braziliensis reveal diversification in the trypanosomatid RNAi pathway. Mol. Microbiol. 2013, 87, 580–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Absalon, S.; Blisnick, T.; Kohl, L.; Toutirais, G.; Dore, G.; Julkowska, D.; Tavenet, A.; Bastin, P. Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol. Biol. Cell 2008, 19, 929–944. [Google Scholar] [CrossRef] [PubMed]
- Beneke, T.; Demay, F.; Hookway, E.; Ashman, N.; Jeffery, H.; Smith, J.; Valli, J.; Becvar, T.; Myskova, J.; Lestinova, T.; et al. Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLoS Pathog. 2019, 15, e1007828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowlkes-Comninellis, T.; Beverley, S.M. Leishmania IFT140 mutants show normal viability but lack external flagella: A tool for the sutdyof flagellar function through the infectious cycle. Cilia 2015, 4 (Suppl. 1), 49. [Google Scholar] [CrossRef] [Green Version]
- Sunter, J.D.; Moreira-Leite, F.; Gull, K. Dependency relationships between IFT-dependent flagellum elongation and cell morphogenesis in Leishmania. Open. Biol. 2018, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madeira da Silva, L.; Owens, K.L.; Murta, S.M.; Beverley, S.M. Regulated expression of the Leishmania major surface virulence factor lipophosphoglycan using conditionally destabilized fusion proteins. Proc. Natl. Acad. Sci. USA 2009, 106, 7583–7588. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Xu, Y.X.; Caradonna, K.L.; Kruzel, E.K.; Burleigh, B.A.; Bangs, J.D.; Hirschberg, C.B. Inhibition of nucleotide sugar transport in Trypanosoma brucei alters surface glycosylation. J. Biol. Chem. 2013, 288, 10599–10615. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Cottrell, T.R.; Pierini, L.M.; Goldman, W.E.; Doering, T.L. RNA interference in the pathogenic fungus Cryptococcus neoformans. Genetics 2002, 160, 463–470. [Google Scholar] [CrossRef]
- Rappleye, C.A.; Engle, J.T.; Goldman, W.E. RNA interference in Histoplasma capsulatum demonstrates a role for α(1,3)-glucan in virulence. Mol. Microbiol. 2004, 53, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Boitz, J.M.; Ullman, B. Adenine and adenosine salvage in Leishmania donovani. Mol. Biochem. Parasitol. 2013, 190, 51–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohl, K.; Zangger, H.; Rossi, M.; Isorce, N.; Lye, L.F.; Owens, K.L.; Beverley, S.M.; Mayer, A.; Fasel, N. Importance of polyphosphate in the Leishmania life cycle. Microb. Cell 2018, 5, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, C.; Veazey, P.; Redmond, S.; Hayes-Sinclair, J.; Chambers, E.; Carrington, M.; Gull, K.; Matthews, K.; Horn, D.; Field, M.C. Chromosome-wide analysis of gene function by RNA interference in the african trypanosome. Eukaryot. Cell 2006, 5, 1539–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brettmann, E.A.; Lye, L.F.; Beverley, S.M. Spontaneous excision and facilitated recovery as a control for phenotypes arising from RNA interference and other dominant transgenes. Mol. Biochem. Parasitol. 2018, 220, 42–45. [Google Scholar] [CrossRef]







| STL Construct | No. Transfectants with WT | No. Transfectants with Δago1− |
|---|---|---|
| PFR1-StL | 243–360 | 360 |
| IFT122-StL (retrograde) | 0 | 460 |
| IFT140-StL (retrograde) | 0 | 265 |
| IFT172-StL (anterograde) | 0 | 289 |
| Negative control | 0 | 0 |
| Gene | Gene ID | Results | Reference |
|---|---|---|---|
| LbrAGO1 | LbrM.11.0360 | Reduced RNAi activity | [10] |
| LbrLPG1 | LbrM.25.0010 | Little mRNA change; remains LPG+ | [10] |
| LbrLPG2 | LbrM.20.2700 | 3-fold lower mRNA; remains LPG+ | [10] |
| LbrLPG3 | LbrM.29.0780 | 3-fold lower mRNA; remains LPG+ | [10] |
| LbrHGPRT | LbrM.21.0990 | Reduced protein | This work |
| LbrPFR1 | LbrM.31.0160 | Reduced mRNA; abnormal swimming | [10] |
| LbrPFR2 | LbrM.16.1480 | Reduced mRNA; abnormal swimming | [10] |
| Leishmania RNA virus 1 | LgyM4147 LbrLEM2700 LbrLEM2780 LbrLEM3874 | LRV1 elimination | [19] |
| Lbr δ Amastin | Lbr.M20.0160 (gene family) | Reduced mRNA; impaired viability of intracellular amastigotes | [21] |
| LgyVTC4 | Lgy4147 VTC4 | Reduced mRNA; polyphosphate levels decreased; little impact on growth | [54] |
| LbrAPRT1 | LbrM.26.0130 | Resistant to APP | This work |
| LbrQDPR | LbrM.20.3970 | Altered drug sensitivity | This work |
| LbrA600 | LbrM.20.3230 | Growth defect as amastigotes | This work |
| LbrIFT140 (retrograde) | LbrM.32.0380 | Unable to recover transfectants of WT Lbr | This work |
| LbrIFT122 (retrograde) | LbrM.35.1120 | Unable to recover transfectants from WT Lbr | This work |
| LbrIFT172 (anterograde) | LbrM.21.1210 | Unable to recover transfectants from WT Lbr | This work |
| Lbr BBS1, BBS3, BBS4 | LbrM.34.4180 LbrM.16.1430 LbrM.35.2520 | No effect on growth or morphology | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lye, L.-F.; Owens, K.L.; Jang, S.; Marcus, J.E.; Brettmann, E.A.; Beverley, S.M. An RNA Interference (RNAi) Toolkit and Its Utility for Functional Genetic Analysis of Leishmania (Viannia). Genes 2023, 14, 93. https://doi.org/10.3390/genes14010093
Lye L-F, Owens KL, Jang S, Marcus JE, Brettmann EA, Beverley SM. An RNA Interference (RNAi) Toolkit and Its Utility for Functional Genetic Analysis of Leishmania (Viannia). Genes. 2023; 14(1):93. https://doi.org/10.3390/genes14010093
Chicago/Turabian StyleLye, Lon-Fye, Katherine L. Owens, Soojin Jang, Joseph E. Marcus, Erin A. Brettmann, and Stephen M. Beverley. 2023. "An RNA Interference (RNAi) Toolkit and Its Utility for Functional Genetic Analysis of Leishmania (Viannia)" Genes 14, no. 1: 93. https://doi.org/10.3390/genes14010093