Relationships between Uncoupling Protein Genes UCP1, UCP2 and UCP3 and Irisin Levels in Residents of the Coldest Region of Siberia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometric Measurements
2.3. Plasma Irisin Analyses
2.4. DNA Analysis
2.5. Search for Signals of Natural Selection
2.6. Statistical Analysis
3. Results
3.1. Plasma Irisin Levels
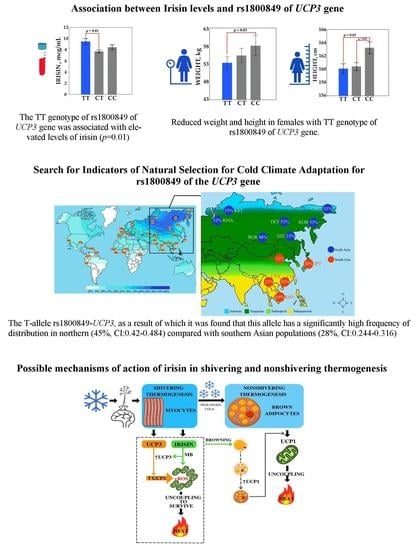
3.2. Association Analysis between Irisin Levels and Six Single Nucleotid Polymorphisms of the Uncoupling Protein Genes
3.3. Search for Signals of Natural Selection for Cold Climate Adaptation for rs1800849 of the UCP3 Gene
4. Discussion
Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haman, F. Shivering in the Cold: From Mechanisms of Fuel Selection to Survival. J. Appl Physiol. 2006, 100, 1702–1708. [Google Scholar] [CrossRef] [PubMed]
- Pant, M.; Bal, N.C.; Periasamy, M. Sarcolipin: A Key Thermogenic and Metabolic Regulator in Skeletal Muscle. Trends. Endocrinol. Metab. 2016, 27, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Aydin, J.; Shabalina, I.G.; Place, N.; Reiken, S.; Zhang, S.-J.; Bellinger, A.M.; Nedergaard, J.; Cannon, B.; Marks, A.R.; Bruton, J.D.; et al. Nonshivering Thermogenesis Protects against Defective Calcium Handling in Muscle. FASEB J. 2008, 22, 3919–3924. [Google Scholar] [CrossRef] [PubMed]
- Bjerregaard, P.; Dewailly, E.; Young, T.K.; Blanchet, C.; Hegele, R.A.; Ebbesson, S.E.O.; Risica, P.M.; Mulvad, G. Blood Pressure among the Inuit (Eskimo) Populations in the Arctic. Scand. J. Public Health 2003, 31, 92–99. [Google Scholar]
- Cannon, B.; Nedergaard, J. Brown Adipose Tissue: Function and Physiological Significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Efremova, A.V. Brown adipose tissue: Main stages of research and potential role in energy balance and obesity. Yakut Med. J. 2019, 65, 92–94. [Google Scholar] [CrossRef]
- Petrovic, N.; Walden, T.B.; Shabalina, I.G.; Timmons, J.A.; Cannon, B.; Nedergaard, J. Chronic Peroxisome Proliferator-Activated Receptor γ (PPARγ) Activation of Epididymally Derived White Adipocyte Cultures Reveals a Population of Thermogenically Competent, UCP1-Containing Adipocytes Molecularly Distinct from Classic Brown Adipocytes. J. Biol. Chem. 2010, 285, 7153–7164. [Google Scholar] [CrossRef]
- Wu, J.; Boström, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.-H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef]
- Vitali, A.; Murano, I.; Zingaretti, M.C.; Frontini, A.; Ricquier, D.; Cinti, S. The Adipose Organ of Obesity-Prone C57BL/6J Mice Is Composed of Mixed White and Brown Adipocytes. J. Lipid Res. 2012, 53, 619–629. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and Beige Fat: Development, Function and Therapeutic Potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional Cloning of the Mouse Obese Gene and Its Human Homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Seale, P.; Kubota, K.; Lunsford, E.; Frangioni, J.V.; Gygi, S.P.; Spiegelman, B.M. Initiation of Myoblast/Brown Fat Switch through a PRDM16-C/EBP-β Transcriptional Complex. Nature 2009, 460, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Bonet, M.L.; Oliver, P.; Palou, A. Pharmacological and Nutritional Agents Promoting Browning of White Adipose Tissue. Biochim. Et Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2013, 1831, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wahl, R. Responses of the Insulin Signaling Pathways in the Brown Adipose Tissue of Rats Following Cold Exposure. PLoS ONE 2014, 9, e99772. [Google Scholar] [CrossRef]
- Cypess, A.M.; Haft, C.R.; Laughlin, M.R.; Hu, H.H. Brown Fat in Humans: Consensus Points and Experimental Guidelines. Cell Metab. 2014, 20, 408–415. [Google Scholar] [CrossRef]
- Suárez-Zamorano, N.; Fabbiano, S.; Chevalier, C.; Stojanović, O.; Colin, D.J.; Stevanović, A.; Veyrat-Durebex, C.; Tarallo, V.; Rigo, D.; Germain, S.; et al. Microbiota Depletion Promotes Browning of White Adipose Tissue and Reduces Obesity. Nat. Med. 2015, 21, 1497–1501. [Google Scholar] [CrossRef]
- Sahuri-Arisoylu, M.; Brody, L.P.; Parkinson, J.R.; Parkes, H.; Navaratnam, N.; Miller, A.D.; Thomas, E.L.; Frost, G.; Bell, J.D. Reprogramming of Hepatic Fat Accumulation and “browning” of Adipose Tissue by the Short-Chain Fatty Acid Acetate. Int. J. Obes. 2016, 40, 955–963. [Google Scholar] [CrossRef]
- Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Browning of White Adipose Tissue: Lessons from Experimental Models. Horm. Mol. Biol. Clin. Investig. 2017, 31, 20160051. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Serino, M.; Blasco-Baque, V.; Azalbert, V.; Barton, R.H.; Cardellini, M.; Latorre, J.; Ortega, F.; Sabater-Masdeu, M.; Burcelin, R.; et al. Gut Microbiota Interacts with Markers of Adipose Tissue Browning, Insulin Action and Plasma Acetate in Morbid Obesity. Mol. Nutr. Food. Res. 2018, 62, 1700721. [Google Scholar] [CrossRef]
- Riley, C.L.; Dao, C.; Kenaston, M.A.; Muto, L.; Kohno, S.; Nowinski, S.M.; Solmonson, A.D.; Pfeiffer, M.; Sack, M.N.; Lu, Z.; et al. The complementary and divergent roles of uncoupling proteins 1 and 3 in thermoregulation. J. Physiol. 2016, 594, 7455–7464. [Google Scholar] [CrossRef]
- Pohl, E.E.; Rupprecht, A.; Macher, G.; Hilse, K.E. Important Trends in UCP3 Investigation. Front. Physiol. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouchani, E.T.; Kazak, L.; Spiegelman, B.M. Mitochondrial reactive oxygen species and adipose tissue thermogenesis: Bridging physiology and mechanisms. J. Biol. Chem. 2017, 292, 16810–16816. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-Dependent Myokine That Drives Brown-Fat-like Development of White Fat and Thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Roca-Rivada, A.; Castelao, C.; Senin, L.L.; Landrove, M.O.; Baltar, J.; Belén Crujeiras, A.; Seoane, L.M.; Casanueva, F.F.; Pardo, M. FNDC5/Irisin Is Not Only a Myokine but Also an Adipokine. PLoS ONE 2013, 8, e60563. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. MRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Smith, S.; Linderman, J.; Courville, A.B.; Brychta, R.J.; Dieckmann, W.; Werner, C.D.; Chen, K.Y.; Celi, F.S. Temperature-Acclimated Brown Adipose Tissue Modulates Insulin Sensitivity in Humans. Diabetes 2014, 63, 3686–3698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin Stimulates Browning of White Adipocytes through Mitogen-Activated Protein Kinase P38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Obruchev, S.V. How I discovered the Pole of Cold in Yakutia. Pioneer 1938, 9, 64–68. [Google Scholar]
- Leonard, W.R.; Snodgrass, J.J.; Sorensen, M.V. Metabolic Adaptation in Indigenous Siberian Populations. Annu. Rev. Anthropol. 2005, 34, 451–471. [Google Scholar] [CrossRef]
- Shephard, R.J.; Lavallée, H. Effects of Enhanced Physical Education on Lung Volumes of Primary School Children. J. Sports Med. Phys. Fit. 1996, 36, 186–194. [Google Scholar]
- Snodgrass, J.J.; Leonard, W.R.; Sorensen, M.V.; Tarskaia, L.A.; Mosher, M.J. The Influence of Basal Metabolic Rate on Blood Pressure among Indigenous Siberians. Am. J. Phys. Anthropol. 2008, 137, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Romanova, A.N.; Klimova, T.M.; Egorova, A.G.; Kuzmina, A.A.; Malogulova, I.S.; Arkhipova, N.S. Prevalence and treatment of arterial hypertension in the native rural population of Yakutia. Yakut Med. J. 2019, 67, 6–9. [Google Scholar] [CrossRef]
- Leonard, W.R.; Levy, S.B.; Tarskaia, L.A.; Klimova, T.M.; Fedorova, V.I.; Baltakhinova, M.E.; Krivoshapkin, V.G.; Snodgrass, J.J. Seasonal Variation in Basal Metabolic Rates among the Yakut (Sakha) of Northeastern Siberia. Am. J. Hum. Biol. 2014, 26, 437–445. [Google Scholar] [CrossRef] [PubMed]
- International Obesity Task Force. Obesity: Managing the Global Epidemic: Report of the World Health Organization (WHO) Consultation; World Health Organization: Geneva, Switzerland, 1997. [Google Scholar]
- Nikanorova, A.A.; Barashkov, N.A.; Pshennikova, V.G.; Nakhodkin, S.S.; Gotovtsev, N.N.; Romanov, G.P.; Solovyev, A.V.; Kuzmina, S.S.; Sazonov, N.N.; Fedorova, S.A. The Role of Nonshivering Thermogenesis Genes on Leptin Levels Regulation in Residents of the Coldest Region of Siberia. Int. J. Mol. Sci. 2021, 22, 4657. [Google Scholar] [CrossRef] [PubMed]
- The 1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 2015, 526, 68–74. [Google Scholar] [CrossRef]
- Stepanov, V.A.; Kharkov, V.N.; Vagaitseva, K.V.; Bocharova, A.V.; Popovich, A.A.; Khitrinskaya, I.Y.; Kazantsev, A.Y. Search for Genetic Markers of Climatic Adaptation in Populations of North Eurasia. Russ. J. Genet. 2017, 53, 1172–1183. [Google Scholar] [CrossRef]
- Madsen, L.; Pedersen, L.M.; Lillefosse, H.H.; Fjaere, E.; Bronstad, I.; Hao, Q.; Petersen, R.K.; Hallenborg, P.; Ma, T.; De Matteis, R.; et al. UCP1 Induction during Recruitment of Brown Adipocytes in White Adipose Tissue Is Dependent on Cyclooxygenase Activity. PLoS ONE 2010, 5, e11391. [Google Scholar] [CrossRef]
- Wang, C.; Bomberg, E.; Billington, C.J.; Levine, A.S.; Kotz, C.M. Brain-Derived Neurotrophic Factor (BDNF) in the Hypothalamic Ventromedial Nucleus Increases Energy Expenditure. Brain Res. 2010, 1336, 66–77. [Google Scholar] [CrossRef]
- Hancock, A.M.; Clark, V.J.; Qian, Y.; Di Rienzo, A. Population Genetic Analysis of the Uncoupling Proteins Supports a Role for UCP3 in Human Cold Resistance. Mol. Biol. Evol. 2011, 28, 601–614. [Google Scholar] [CrossRef]
- Bianco, A.C.; McAninch, E.A. The Role of Thyroid Hormone and Brown Adipose Tissue in Energy Homoeostasis. Lancet Diabetes Endocrinol. 2013, 1, 250–258. [Google Scholar] [CrossRef]
- Irving, B.A.; Still, C.D.; Argyropoulos, G. Does IRISIN Have a BRITE Future as a Therapeutic Agent in Humans? Curr. Obes. Rep. 2014, 3, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kajimura, S.; Saito, M. A New Era in Brown Adipose Tissue Biology: Molecular Control of Brown Fat Development and Energy Homeostasis. Annu. Rev. Physiol. 2014, 76, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, E.O.; Mayorov, A.Y.; Shestakova, M.V.; Dedov, I.I. Metabolic characteristics and therapeutic potential of brown and “beige” adipose tissues. Diabetes Mellit. 2014, 17, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Sazzini, M.; Schiavo, G.; De Fanti, S.; Martelli, P.L.; Casadio, R.; Luiselli, D. Searching for Signatures of Cold Adaptations in Modern and Archaic Humans: Hints from the Brown Adipose Tissue Genes. Heredity 2014, 113, 259–267. [Google Scholar] [CrossRef]
- Reynés, B.; García-Ruiz, E.; Oliver, P.; Palou, A. Gene Expression of Peripheral Blood Mononuclear Cells Is Affected by Cold Exposure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R824–R834. [Google Scholar] [CrossRef]
- Al-Daghri, N.M.; Alkharfy, K.M.; Rahman, S.; Amer, O.E.; Vinodson, B.; Sabico, S.; Piya, M.K.; Harte, A.L.; McTernan, P.G.; Alokail, M.S.; et al. Irisin as a Predictor of Glucose Metabolism in Children: Sexually Dimorphic Effects. Eur. J. Clin. Invest. 2014, 44, 119–124. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Saridakis, Z.G.; Kynigopoulos, G.; Skouvaklidou, E.C.; Molyvas, D.; Vasiloglou, M.F.; Apostolou, A.; Karagiozoglou-Lampoudi, T.; Siopi, A.; et al. Circulating Irisin in Healthy, Young Individuals: Day-Night Rhythm, Effects of Food Intake and Exercise, and Associations with Gender, Physical Activity, Diet, and Body Composition. J. Clin. Endocrinol. Metab. 2014, 99, 3247–3255. [Google Scholar] [CrossRef]
- Ruan, Q.; Huang, Y.; Yang, L.; Ruan, J.; Gu, W.; Zhang, X.; Zhang, Y.; Zhang, W.; Yu, Z. The Effects of Both Age and Sex on Irisin Levels in Paired Plasma and Cerebrospinal Fluid in Healthy Humans. Peptides 2019, 113, 41–51. [Google Scholar] [CrossRef]
- Liu, J.-J.; Wong, M.D.S.; Toy, W.C.; Tan, C.S.H.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower Circulating Irisin Is Associated with Type 2 Diabetes Mellitus. J. Diabetes Its. Complicat. 2013, 27, 365–369. [Google Scholar] [CrossRef]
- Jameel, F.; Thota, R.N.; Wood, L.G.; Plunkett, B.; Garg, M.L. Sex-Dependent Association between Circulating Irisin Levels and Insulin Resistance in Healthy Adults. J. Nutr. Intermed. Metab. 2015, 2, 86–92. [Google Scholar] [CrossRef]
- Pardo, M.; Crujeiras, A.B.; Amil, M.; Aguera, Z.; Jiménez-Murcia, S.; Baños, R.; Botella, C.; de la Torre, R.; Estivill, X.; Fagundo, A.B.; et al. Association of Irisin with Fat Mass, Resting Energy Expenditure, and Daily Activity in Conditions of Extreme Body Mass Index. Int. J. Endocrinol. 2014, 2014, 857270. [Google Scholar] [CrossRef] [PubMed]
- Perakakis, N.; Triantafyllou, G.A.; Fernández-Real, J.M.; Huh, J.Y.; Park, K.H.; Seufert, J.; Mantzoros, C.S. Physiology and Role of Irisin in Glucose Homeostasis. Nat. Rev. Endocrinol. 2017, 13, 324–337. [Google Scholar] [CrossRef]
- Sahin-Efe, A.; Upadhyay, J.; Ko, B.-J.; Dincer, F.; Park, K.H.; Migdal, A.; Vokonas, P.; Mantzoros, C. Irisin and Leptin Concentrations in Relation to Obesity, and Developing Type 2 Diabetes: A Cross Sectional and a Prospective Case-Control Study Nested in the Normative Aging Study. Metabolism 2018, 79, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin Is Expressed and Produced by Human Muscle and Adipose Tissue in Association With Obesity and Insulin Resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Klangjareonchai, T.; Nimitphong, H.; Saetung, S.; Bhirommuang, N.; Samittarucksa, R.; Chanprasertyothin, S.; Sudatip, R.; Ongphiphadhanakul, B. Circulating Sclerostin and Irisin Are Related and Interact with Gender to Influence Adiposity in Adults with Prediabetes. Int. J. Endocrinol. 2014, 2014, 261545. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Han, F.; Sun, X. The Relationship between Circulating Irisin Levels and Endothelial Function in Lean and Obese Subjects. Clin. Endocrinol. 2015, 83, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Sanchis-Gomar, F.; Lippi, G.; Mayero, S.; Perez-Quilis, C.; García-Giménez, J.L. Irisin: A New Potential Hormonal Target for the Treatment of Obesity and Type 2 Diabetes. J. Diabetes 2012, 4, 196. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zhang, R.; Jiang, F.; Wang, J.; Chen, M.; Peng, D.; Yan, J.; Wang, S.; Bao, Y.; Hu, C.; et al. Circulating Irisin Levels Are Associated with Lipid and Uric Acid Metabolism in a Chinese Population. Clin. Exp. Pharmacol. Physiol. 2015, 42, 896–901. [Google Scholar] [CrossRef]
- D’Amuri, A.; Raparelli, V.; Sanz, J.M.; Capatti, E.; Di Vece, F.; Vaccari, F.; Lazzer, S.; Zuliani, G.; Dalla Nora, E.; Neri, L.M.; et al. Biological Response of Irisin Induced by Different Types of Exercise in Obese Subjects: A Non-Inferiority Controlled Randomized Study. Biology 2022, 11, 392. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.; Wang, Y.; Cao, R.; Zhang, Z.; Fu, L. What Is the Relationship Between Body Mass Index, Sex Hormones, Leptin, and Irisin in Children and Adolescents? A Path Analysis. Front. Pediatr. 2022, 10, 823424. [Google Scholar] [CrossRef]
- Xu, Y.-P.; Liang, L.; Wang, C.-L.; Fu, J.-F.; Liu, P.-N.; Lv, L.-Q.; Zhu, Y.-M. Association between UCP3 Gene Polymorphisms and Nonalcoholic Fatty Liver Disease in Chinese Children. World J. Gastroenterol. 2013, 19, 5897–5903. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Xia, J.; Bogardus, C.; Pratley, R.E.; Ravussin, E. Skeletal Muscle Uncoupling Protein 3 Expression Is a Determinant of Energy Expenditure in Pima Indians. Diabetes 1999, 48, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Schrauwen, P.; Xia, J.; Walder, K.; Snitker, S.; Ravussin, E. A Novel Polymorphism in the Proximal UCP3 Promoter Region: Effect on Skeletal Muscle UCP3 MRNA Expression and Obesity in Male Non-Diabetic Pima Indians. Int. J. Obes. 1999, 23, 1242–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acín, A.; Rodriguez, M.; Rique, H.; Canet, E.; Boutin, J.A.; Galizzi, J.P. Cloning and Characterization of the 5’ Flanking Region of the Human Uncoupling Protein 3 (UCP3) Gene. Biochem. Biophys. Res. Commun. 1999, 258, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Boss, O.; Samec, S.; Paoloni-Giacobino, A.; Rossier, C.; Dulloo, A.; Seydoux, J.; Muzzin, P.; Giacobino, J.P. Uncoupling Protein-3: A New Member of the Mitochondrial Carrier Family with Tissue-Specific Expression. FEBS Lett. 1997, 408, 39–42. [Google Scholar] [CrossRef]
- Vidal-Puig, A.; Solanes, G.; Grujic, D.; Flier, J.S.; Lowell, B.B. UCP3: An Uncoupling Protein Homologue Expressed Preferentially and Abundantly in Skeletal Muscle and Brown Adipose Tissue. Biochem. Biophys. Res. Commun. 1997, 235, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Solanes, G.; Vidal-Puig, A.; Grujic, D.; Flier, J.S.; Lowell, B.B. The Human Uncoupling Protein-3 Gene. Genomic Structure, Chromosomal Localization, and Genetic Basis for Short and Long Form Transcripts. J. Biol. Chem. 1997, 272, 25433–25436. [Google Scholar] [CrossRef] [PubMed]
- Liebig, M.; von Praun, C.; Heldmaier, G.; Klingenspor, M. Absence of UCP3 in Brown Adipose Tissue Does Not Impair Nonshivering Thermogenesis. Physiol. Biochem. Zool. 2004, 77, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Hilse, K.E.; Kalinovich, A.V.; Rupprecht, A.; Smorodchenko, A.; Zeitz, U.; Staniek, K.; Erben, R.G.; Pohl, E.E. The Expression of UCP3 Directly Correlates to UCP1 Abundance in Brown Adipose Tissue. Biochim. Biophys. Acta 2016, 1857, 72–78. [Google Scholar] [CrossRef]
- Zackova, M.; Skobisová, E.; Urbánková, E.; Jezek, P. Activating Omega-6 Polyunsaturated Fatty Acids and Inhibitory Purine Nucleotides Are High Affinity Ligands for Novel Mitochondrial Uncoupling Proteins UCP2 and UCP3. J. Biol. Chem. 2003, 278, 20761–20769. [Google Scholar] [CrossRef]
- Macher, G.; Koehler, M.; Rupprecht, A.; Kreiter, J.; Hinterdorfer, P.; Pohl, E.E. Inhibition of Mitochondrial UCP1 and UCP3 by Purine Nucleotides and Phosphate. Biochim. Biophys. Acta Biomembr. 2018, 1860, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Nègre-Salvayre, A.; Hirtz, C.; Carrera, G.; Cazenave, R.; Troly, M.; Salvayre, R.; Pénicaud, L.; Casteilla, L. A Role for Uncoupling Protein-2 as a Regulator of Mitochondrial Hydrogen Peroxide Generation. FASEB J. 1997, 11, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress. Antioxidants 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Tekin, S.; Erden, Y.; Ozyalin, F.; Onalan, E.E.; Cigremis, Y.; Colak, C.; Tekedereli, I.; Sandal, S. Central Irisin Administration Suppresses Thyroid Hormone Production but Increases Energy Consumption in Rats. Neurosci. Lett. 2018, 674, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Gannon, N.P.; Barberena, M.A.; Garcia-Smith, R.; Bisoffi, M.; Mermier, C.M.; Conn, C.A.; Trujillo, K.A. Characterization of the Metabolic Effects of Irisin on Skeletal Muscle in Vitro. Diabetes Obes. Metab. 2014, 16, 711–718. [Google Scholar] [CrossRef]
- Sawicka-Gutaj, N.; Erampamoorthy, A.; Zybek-Kocik, A.; Kyriacou, A.; Zgorzalewicz-Stachowiak, M.; Czarnywojtek, A.; Ruchała, M. The Role of Thyroid Hormones on Skeletal Muscle Thermogenesis. Metabolites 2022, 12, 336. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan–Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef]
- Vidal-Puig, A.J.; Grujic, D.; Zhang, C.Y.; Hagen, T.; Boss, O.; Ido, Y.; Szczepanik, A.; Wade, J.; Mootha, V.; Cortright, R.; et al. Energy Metabolism in Uncoupling Protein 3 Gene Knockout Mice. J. Biol. Chem. 2000, 275, 16258–16266. [Google Scholar] [CrossRef]
- Brand, M.D.; Pamplona, R.; Portero-Otín, M.; Requena, J.R.; Roebuck, S.J.; Buckingham, J.A.; Clapham, J.C.; Cadenas, S. Oxidative Damage and Phospholipid Fatty Acyl Composition in Skeletal Muscle Mitochondria from Mice Underexpressing or Overexpressing Uncoupling Protein 3. Biochem. J. 2002, 368, 597–603. [Google Scholar] [CrossRef]
- Zhao, R.-Z.; Jiang, S.; Zhang, L.; Yu, Z.-B. Mitochondrial Electron Transport Chain, ROS Generation and Uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Brand, M.D. Uncoupling to Survive? The Role of Mitochondrial Inefficiency in Ageing. Exp. Gerontol. 2000, 35, 811–820. [Google Scholar] [CrossRef]




| Characteristics | Underweight (n = 36) | p 1 | Normal Weight (n = 214) | p2 | Overweight and Obese (n = 29) | p 1 | |||
|---|---|---|---|---|---|---|---|---|---|
| F (n = 25) | M (n = 11) | F (n = 144) | M (n = 70) | F (n = 16) | M (n = 13) | ||||
| Weight (kg) | 44.88 ± 3.71 | 50.45 ± 3.42 | 0.01 | 55.53 ± 5.8 | 66.19 ± 7.44 | 0.01 | 72.75 ± 11.13 | 81.46 ± 8.3 | 0.01 |
| Height (cm) | 160.24 ± 5.14 | 170.36 ± 5.89 | 0.01 | 160.92 ± 6.03 | 173.33 ± 5.98 | 0.01 | 162.19 ± 4.96 | 174.69 ± 6.64 | 0.01 |
| BMI (kg/m2) | 17.45 ± 0.73 | 17.39 ± 0.91 | 0.868 | 21.42 ± 1.62 | 22 ± 1.89 | 0.03 | 27.56 ± 2.88 | 26.64 ± 1.49 | 0.539 |
| Irisin (mcg/mL) | 7.88 ± 1.96 | 8.52 ± 2.64 | 0.904 | 8.42 ± 2.92 | 7.51 ± 1.61 | 0.02 | 8.27 ± 1.96 | 8.48 ± 2.16 | 0.965 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikanorova, A.A.; Barashkov, N.A.; Pshennikova, V.G.; Gotovtsev, N.N.; Romanov, G.P.; Solovyev, A.V.; Kuzmina, S.S.; Sazonov, N.N.; Fedorova, S.A. Relationships between Uncoupling Protein Genes UCP1, UCP2 and UCP3 and Irisin Levels in Residents of the Coldest Region of Siberia. Genes 2022, 13, 1612. https://doi.org/10.3390/genes13091612
Nikanorova AA, Barashkov NA, Pshennikova VG, Gotovtsev NN, Romanov GP, Solovyev AV, Kuzmina SS, Sazonov NN, Fedorova SA. Relationships between Uncoupling Protein Genes UCP1, UCP2 and UCP3 and Irisin Levels in Residents of the Coldest Region of Siberia. Genes. 2022; 13(9):1612. https://doi.org/10.3390/genes13091612
Chicago/Turabian StyleNikanorova, Alena A., Nikolay A. Barashkov, Vera G. Pshennikova, Nyurgun N. Gotovtsev, Georgii P. Romanov, Aisen V. Solovyev, Sargylana S. Kuzmina, Nikolay N. Sazonov, and Sardana A. Fedorova. 2022. "Relationships between Uncoupling Protein Genes UCP1, UCP2 and UCP3 and Irisin Levels in Residents of the Coldest Region of Siberia" Genes 13, no. 9: 1612. https://doi.org/10.3390/genes13091612